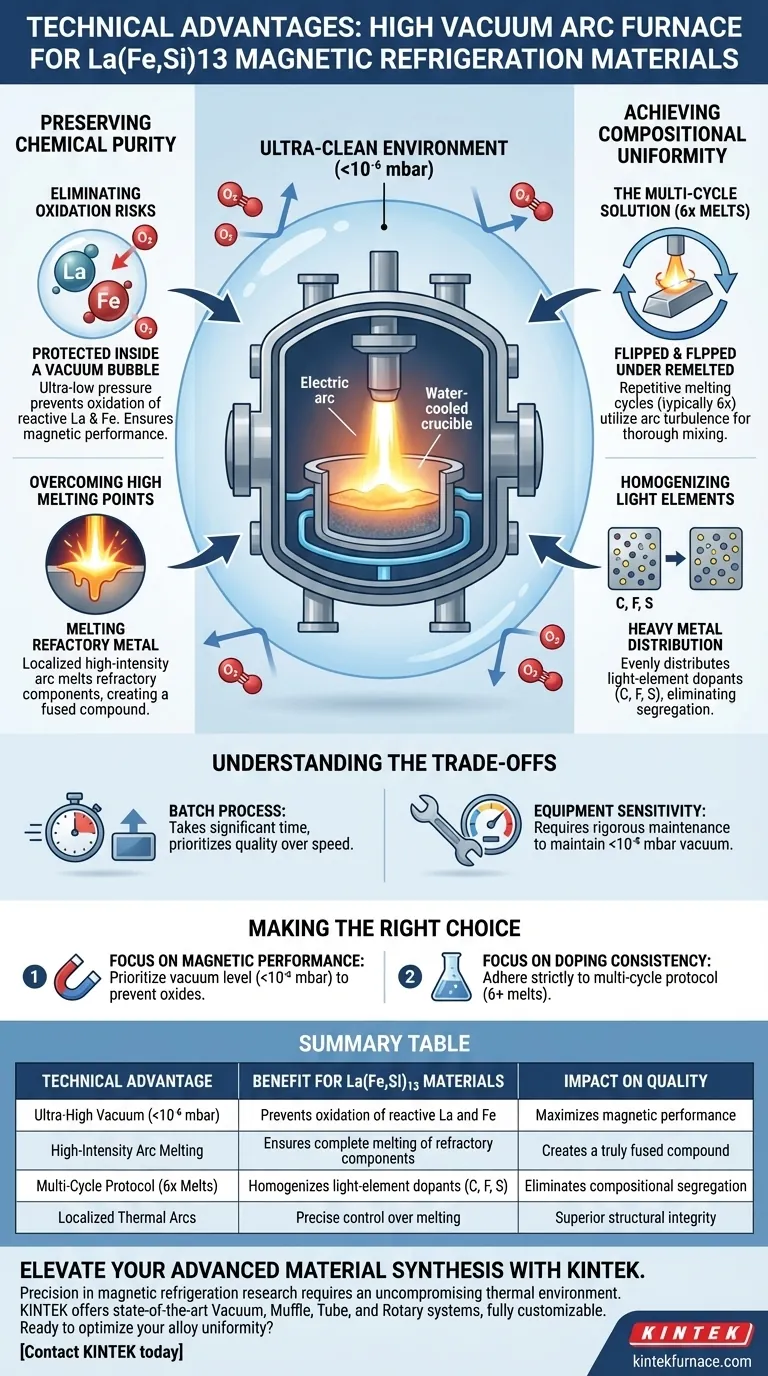
The primary technical advantage of a high vacuum arc furnace is its ability to synthesize high-purity alloys by operating in an ultra-clean environment with pressures below $10^{-6}$ mbar. This specific vacuum level is critical for preventing the oxidation of reactive raw materials, specifically Lanthanum (La) and Iron (Fe), while the furnace's high-temperature arc capability ensures the complete melting of refractory metals.
The high vacuum arc furnace solves the dual challenges of chemical oxidation and compositional segregation. By combining a strictly controlled ultra-low pressure environment with high-intensity thermal arcs and repetitive melting cycles, it guarantees the structural integrity required for effective magnetic refrigeration materials.

Preserving Chemical Purity
Eliminating Oxidation Risks
The synthesis of La(Fe,Si)13-based materials involves highly reactive elements. Lanthanum and Iron are particularly susceptible to oxidation when exposed to trace amounts of oxygen at high temperatures.
A high vacuum arc furnace mitigates this by maintaining a vacuum level better than $10^{-6}$ mbar. This creates a near-pristine environment that protects the active raw materials, ensuring that the magnetic properties of the final alloy are not degraded by oxide impurities.
Overcoming High Melting Points
Magnetic refrigeration alloys often contain components with vastly different melting points. Standard heating methods may struggle to fully liquefy the most refractory elements without overheating others.
The vacuum arc generates intense, localized heat. This ensures that even high-melting-point metals are fully melted and integrated into the alloy matrix, creating a truly fused compound rather than a sintered mixture.
Achieving Compositional Uniformity
The Challenge of Light-Element Doping
To optimize these materials for refrigeration, they are often modified with light elements such as Carbon, Fluorine, or Sulfur. Introducing these dopants into a heavy metal matrix can lead to segregation, where the elements do not mix evenly.
The Multi-Cycle Solution
Uniformity is achieved through a specific process protocol inherent to arc melting: multiple melting cycles.
Standard practice typically involves re-melting the ingot approximately six times. This repetitive process utilizes the arc's turbulence to thoroughly mix the multi-component alloy. The result is a high degree of chemical composition uniformity, ensuring that the light-element dopants are evenly distributed throughout the material.
Understanding the Trade-offs
Process Intensity vs. Throughput
While the high vacuum arc furnace offers superior quality, it is a batch-process technique that requires significant time per unit.
The requirement for multiple melting cycles (typically six) to achieve homogeneity acts as a bottleneck. Unlike continuous casting methods, this approach prioritizes material quality over production speed.
Equipment Sensitivity
Achieving pressures below $10^{-6}$ mbar requires rigorous equipment maintenance. Any leak or pump failure that compromises the vacuum level will result in immediate oxidation of the Lanthanum, rendering the batch unusable.
Making the Right Choice for Your Goal
To maximize the effectiveness of a high vacuum arc furnace for your specific material needs, consider the following:
- If your primary focus is Magnetic Performance: Prioritize the vacuum level ($<10^{-6}$ mbar) above all else to prevent oxides from disrupting the magnetic domains.
- If your primary focus is Doping Consistency: Adhere strictly to the multi-cycle protocol (minimum 6 melts) to ensure light elements like Carbon or Sulfur are fully homogenized.
By strictly controlling the vacuum environment and melting repetition, you convert raw volatility into precise, high-performance material stability.
Summary Table:
| Technical Advantage | Benefit for La(Fe,Si)13 Materials | Impact on Quality |
|---|---|---|
| Ultra-High Vacuum (<10⁻⁶ mbar) | Prevents oxidation of reactive La and Fe | Maximizes magnetic performance |
| High-Intensity Arc Melting | Ensures complete melting of refractory components | Creates a truly fused compound |
| Multi-Cycle Protocol (6x Melts) | Homogenizes light-element dopants (C, F, S) | Eliminates compositional segregation |
| Localized Thermal Arcs | Precise control over melting of multi-component alloys | Superior structural integrity |
Elevate Your Advanced Material Synthesis with KINTEK
Precision in magnetic refrigeration research requires an uncompromising thermal environment. Backed by expert R&D and manufacturing, KINTEK offers state-of-the-art Vacuum, Muffle, Tube, and Rotary systems, all fully customizable to meet the rigorous demands of your lab. Whether you are doping sensitive light elements or melting refractory alloys, our high-temp furnaces provide the stability and purity your materials deserve.
Ready to optimize your alloy uniformity? Contact KINTEK today to discuss your custom furnace requirements.
Visual Guide
References
- Fengqi Zhang, Yang Ren. Engineering Light‐Element Modified LaFe <sub>11.6</sub> Si <sub>1.4</sub> Compounds Enables Tunable Giant Magnetocaloric Effect. DOI: 10.1002/advs.202416288
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- Why is a Vacuum Induction Melting (VIM) furnace essential? Secure Superalloy Purity and Performance
- What are the advantages of using a vacuum casting furnace? Achieve Purity and Precision in Metal Processing
- How do vacuum induction furnaces facilitate titanium hydride dehydrogenation? Achieve High-Purity Metal Powder
- Why is a double-layer water-cooled stainless steel chamber used in equipment for preparing ultrafine magnesium powder via the evaporation-condensation method?
- What is the role of the quartz nozzle in half-Heusler induction melting? Optimize Alloy Ribbon Precision
- How does IGBT induction furnace technology enhance performance? Achieve Superior Melting Efficiency & Control
- What is the role of a Vacuum Induction Melting Furnace in chromium-steel prep? Secure Purity & Composition Control
- What is vacuum arc melting (VAR) and what is its primary purpose? Achieve Superior Metal Purity and Performance



















