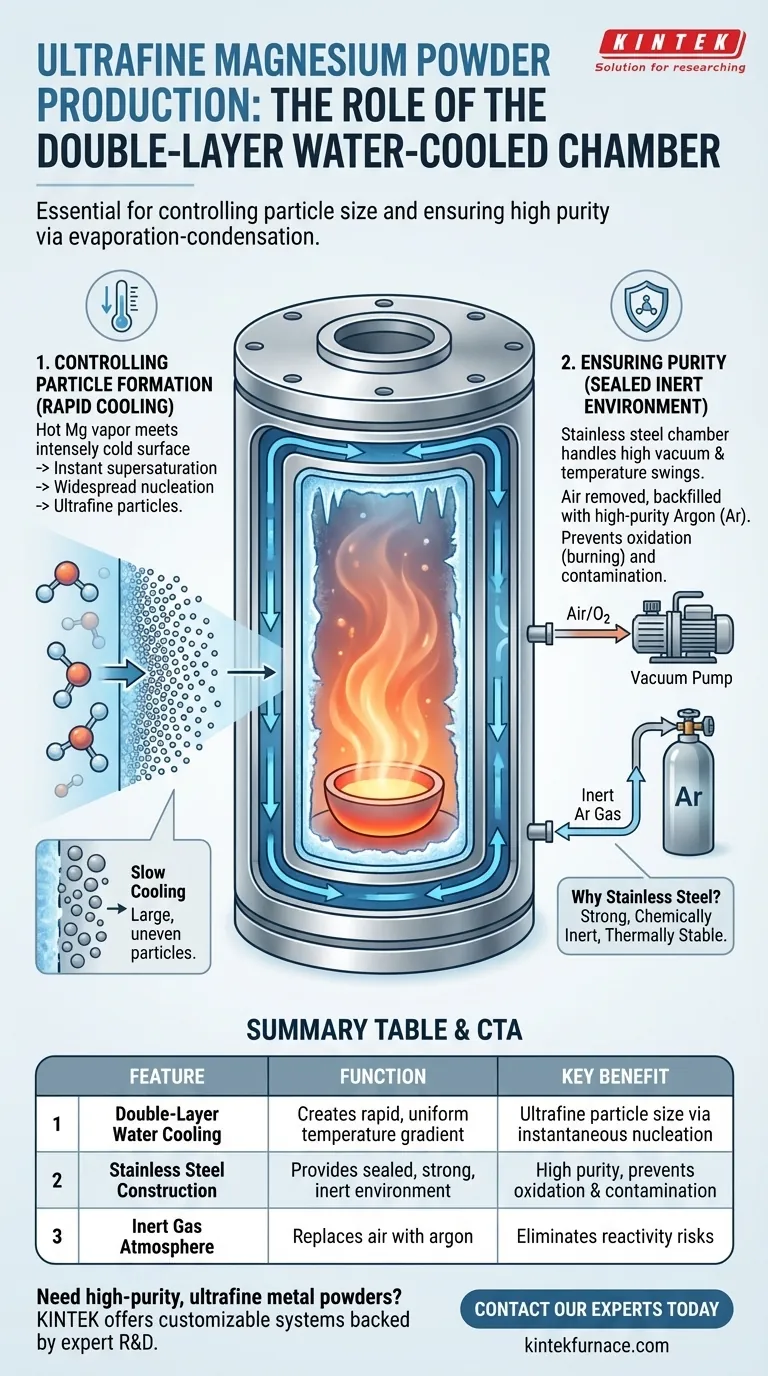
In short, the double-layer water-cooled stainless steel chamber is essential for two reasons: it creates a cold surface for the hot magnesium vapor to rapidly condense into ultrafine particles, and it provides a sealed, clean environment that prevents the highly reactive magnesium from catching fire or becoming contaminated. This dual-function design is the cornerstone of producing high-purity, nano-scale magnesium powder using this method.
The chamber's design isn't arbitrary; it's a carefully engineered solution to control the two most critical variables in the process: the temperature gradient that dictates particle size and the atmosphere that guarantees product purity.

Controlling Particle Formation: The Role of Cooling
The primary goal is to create "ultrafine" powder, not just a solid block of magnesium. This requires precise control over the transition from gas to solid, which is managed entirely by the chamber's cooling system.
From Vapor to Solid
The process begins by heating solid magnesium until it turns into a high-temperature vapor. To turn it back into a solid powder, this vapor must be cooled on a surface.
Achieving "Ultrafine" Size
The key to creating tiny, "ultrafine" particles is rapid cooling. When the hot magnesium vapor makes contact with the chamber's intensely cold inner wall, the dramatic temperature difference forces it to become supersaturated almost instantly. This rapid change triggers widespread nucleation, where countless microscopic particles form simultaneously, rather than allowing a few particles to grow large.
The Double-Layer Design
A double-layer "jacket" design is the most effective way to maintain a uniformly cold inner surface. Chilled water is constantly circulated through the space between the two layers, efficiently pulling heat away and ensuring the entire condensation area remains at the required low temperature.
Ensuring Purity: The Role of the Sealed Chamber
Magnesium is highly reactive, especially at the high temperatures required for evaporation. Any exposure to oxygen would result in immediate oxidation (effectively, burning), ruining the product. The chamber's material and construction prevent this.
The High Reactivity of Magnesium
At elevated temperatures, magnesium vapor will aggressively react with oxygen and other elements in the air. The stainless steel chamber acts as a sealed barrier against the outside atmosphere.
Creating an Inert Atmosphere
Before the process begins, the chamber is sealed and a high vacuum is pulled to remove virtually all the air. The chamber is then backfilled with a high-purity inert gas, typically argon. This creates a completely non-reactive environment for the magnesium to evaporate and condense in, ensuring the final powder is pure magnesium.
Why Stainless Steel?
Stainless steel is the ideal material for this application. It is strong enough to handle high vacuum without collapsing, is chemically inert so it won't contaminate the magnesium, and it withstands the significant temperature swings of the process.
Making the Right Choice for Your Goal
The chamber's design directly enables control over the final product's characteristics. Understanding which feature controls which outcome is crucial for process optimization.
- If your primary focus is minimizing particle size: The key is to maximize the temperature gradient. This means ensuring your cooling system is highly efficient and can maintain the lowest possible wall temperature.
- If your primary focus is maximizing product purity: The key is the integrity of your sealed environment. This demands a high-quality vacuum system, leak-proof seals, and the use of ultra-high purity inert gas.
Ultimately, successful ultrafine powder synthesis depends on equipment designed to precisely manipulate both physical and chemical conditions.
Summary Table:
| Chamber Feature | Function | Key Benefit |
|---|---|---|
| Double-Layer Water Cooling | Creates a rapid, uniform temperature gradient | Forces instantaneous nucleation for ultrafine particle size |
| Stainless Steel Construction | Provides a sealed, strong, and inert environment | Prevents oxidation and contamination, ensuring high purity |
| Inert Gas Atmosphere | Replaces air with argon or other inert gases | Eliminates reactivity risks during evaporation and condensation |
Need to produce high-purity, ultrafine metal powders? The right equipment is critical for controlling particle size and preventing contamination. Backed by expert R&D and manufacturing, KINTEK offers customizable Muffle, Tube, Rotary, Vacuum, and CVD systems, including specialized chambers for evaporation-condensation processes. Let us help you optimize your synthesis for superior results. Contact our experts today to discuss your specific requirements!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process



















