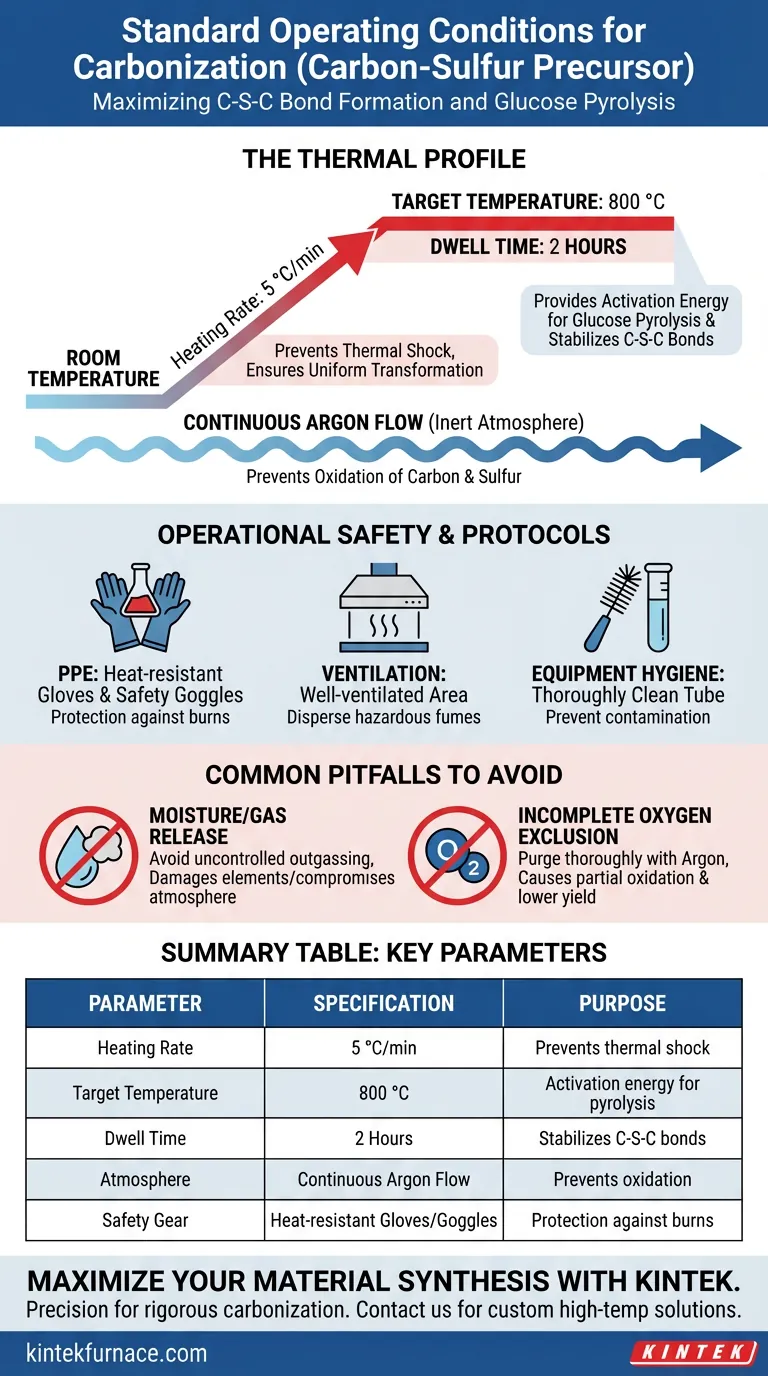
The standard operating conditions for carbonizing a carbon-sulfur precursor involve a controlled heating rate of 5 °C/min rising from room temperature to a target of 800 °C, where it is held for 2 hours. To prevent material oxidation during this process, the tube furnace must maintain a stable inert atmosphere, typically using a continuous argon flow.
The critical objective of this thermal profile is not just carbonization, but the specific promotion of thermal pyrolysis of glucose and the formation of stable C-S-C chemical bonds. This structure anchors the sulfur to the carbon framework, a process that requires precise temperature control and total exclusion of oxygen.

Configuring the Thermal Profile
The Ramping Phase
The furnace should be set to rise from room temperature at a rate of 5 °C/min.
This moderate ramp rate prevents thermal shock to the precursor material. It ensures that the chemical transformation occurs uniformly throughout the sample volume.
Target Temperature and Dwell Time
Once the furnace reaches 800 °C, it must maintain this temperature for a duration of 2 hours.
This dwell time provides the necessary activation energy to complete the pyrolysis of glucose. It is during this phase that the critical C-S-C bonds are stabilized, integrating sulfur into the carbon matrix.
Atmosphere Control
Throughout the entire heating and cooling cycle, an inert argon flow is non-negotiable.
This inert environment shields the precursor from oxygen. Without this shield, the high temperatures would immediately oxidize the carbon and sulfur, destroying the material rather than synthesizing it.
Operational Safety and Protocols
Personal Protective Equipment (PPE)
Operators must strictly adhere to safety protocols, including wearing heat-resistant gloves and safety goggles.
High-temperature furnaces present significant burn hazards. Direct contact with the tube or sample boats during unloading can cause severe injury without proper protection.
Ventilation and Environment
The furnace must be operated in a well-ventilated area.
The carbonization process can release hazardous fumes or volatile byproducts. Proper ventilation ensures these gases are safely dispersed away from the operator.
Equipment Hygiene
The furnace tube must be thoroughly cleaned before every use.
Residue from previous experiments can act as contaminants, altering the chemical reaction. A clean environment is essential for maintaining the purity and stoichiometric accuracy of the final product.
Common Pitfalls to Avoid
Moisture and Gas Release
Avoid loading materials that release significant amounts of moisture or uncontrolled gases upon heating.
Excessive outgassing can destabilize the internal pressure of the tube or damage the heating elements. It may also compromise the purity of the inert argon atmosphere.
Incomplete Oxygen Exclusion
Failing to purge the tube completely with argon before heating is a frequent error.
Even trace amounts of oxygen remaining in the tube can lead to partial oxidation. This results in a lower yield and a degraded structural framework for the carbon-sulfur composite.
Making the Right Choice for Your Goal
To ensure the successful synthesis of your carbon-sulfur precursor, align your procedure with your specific outcome:
- If your primary focus is Chemical Stability: prioritize the 2-hour hold at 800 °C, as this duration is critical for forming the robust C-S-C bonds that stabilize the material.
- If your primary focus is Material Purity: ensure the argon flow is continuous and stable and that the furnace tube is meticulously cleaned to prevent oxidation and cross-contamination.
Precision in your thermal ramp and strict adherence to atmospheric controls are the defining factors between a failed experiment and a high-performance precursor.
Summary Table:
| Parameter | Specification | Purpose |
|---|---|---|
| Heating Rate | 5 °C/min | Prevents thermal shock; ensures uniform transformation |
| Target Temperature | 800 °C | Provides activation energy for glucose pyrolysis |
| Dwell Time | 2 Hours | Stabilizes C-S-C chemical bonds |
| Atmosphere | Continuous Argon Flow | Prevents oxidation of carbon and sulfur |
| Safety Gear | Heat-resistant Gloves/Goggles | Protection against high-temperature burns |
Maximize Your Material Synthesis with KINTEK
Precision is non-negotiable when forming critical C-S-C chemical bonds. At KINTEK, we empower researchers and manufacturers with high-performance thermal solutions designed for rigorous carbonization processes.
Our Advantage to You:
- Expert R&D & Manufacturing: High-temp systems engineered for stability.
- Fully Customizable: Tailored Muffle, Tube, Rotary, Vacuum, and CVD systems for your unique specs.
- Total Control: Achieve the precise 800 °C profiles and inert environments your research demands.
Ready to elevate your lab's efficiency? Contact us today to discuss your custom furnace needs!
Visual Guide
References
- Yaoping Guo, Rui Fang. Sulfur-doped activated carbon for the efficient degradation of tetracycline with persulfate: Insight into the effect of pore structure on catalytic performance. DOI: 10.1039/d3ra08958d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What is a tube furnace and where is it commonly used? Discover Precision Heating for Advanced Materials
- Why is a tube furnace equipped with an ammonia flow control system necessary for GaN nanopowder synthesis?
- What is the working principle of a fluidized bed vertical tube furnace? Achieve Superior Heat Treatment Efficiency
- What is the academic use of drop tube furnaces? Unlock Precise High-Temp Research for Materials and Energy
- What is the primary function of a tube furnace in Leidenfrost experiments? Preheating with Precision & Protection
- What industries commonly use tube furnaces? Essential for High-Tech Materials and Electronics
- How does a two-stage sintering process in a tube furnace contribute to high-performance sodium-ion battery cathodes?
- How does the choice of liner material for a laboratory packed-bed tubular reactor impact CO2 hydrogenation experiments?



















