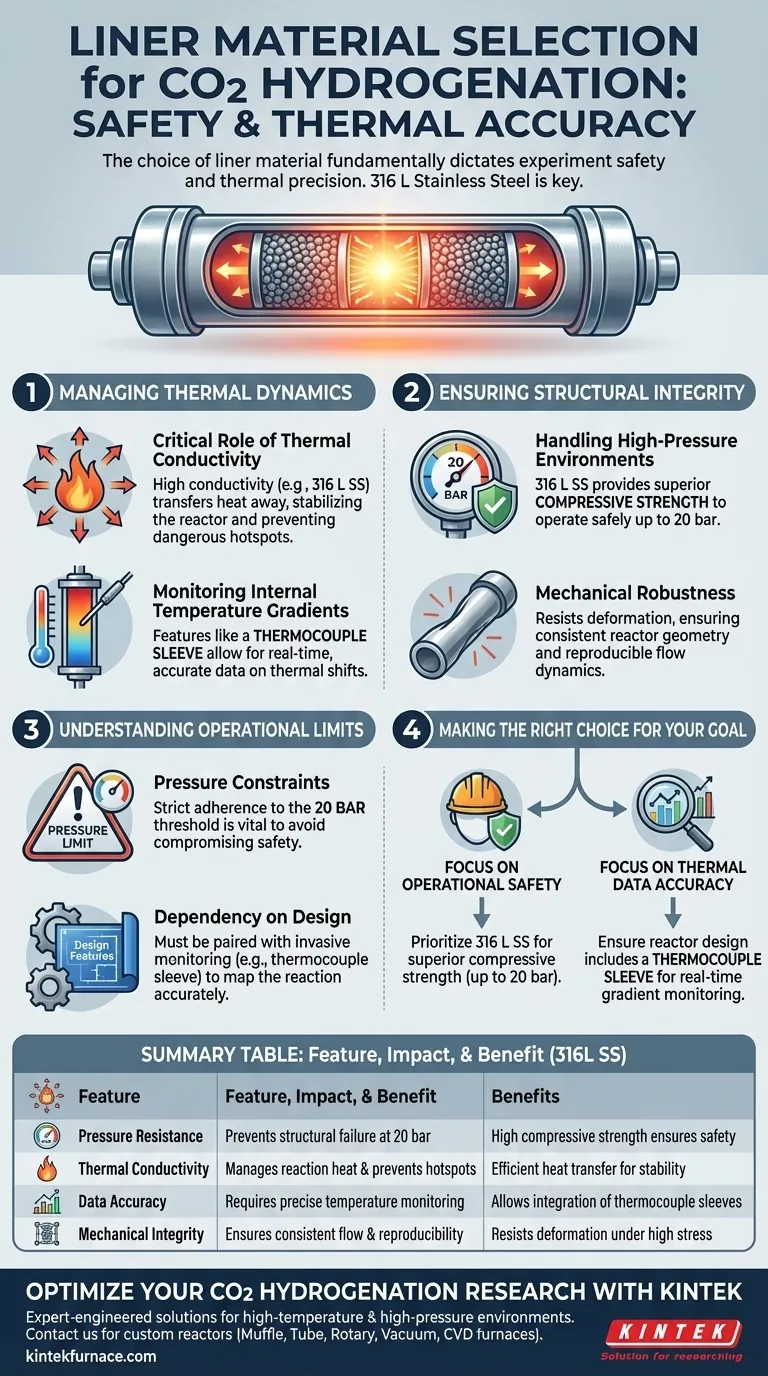
The choice of liner material fundamentally dictates the safety and thermal accuracy of your experiment. Specifically, utilizing materials like 316 L stainless steel ensures the reactor can withstand pressures up to 20 bar while effectively managing the significant thermal shifts associated with carbon dioxide hydrogenation.
Carbon dioxide hydrogenation generates significant heat and requires high pressure; the liner material must balance superior compressive strength with high thermal conductivity to prevent structural failure and thermal runaway.
Managing Thermal Dynamics
The Critical Role of Thermal Conductivity
Carbon dioxide hydrogenation is characterized by significant thermal shifts. To manage this, you must select a liner material with high thermal conductivity, such as 316 L stainless steel.
High conductivity facilitates the transfer of heat away from the reaction site. This helps stabilize the reactor environment and prevents dangerous hotspots within the catalyst bed.
Monitoring Internal Temperature Gradients
Material choice alone is not enough; the reactor design must leverage the material's properties for data visibility.
A well-designed stainless steel reactor includes features like a thermocouple sleeve. This allows for real-time, accurate monitoring of temperature gradients across the bed, ensuring you capture precise data on thermal shifts.
Ensuring Structural Integrity
Handling High-Pressure Environments
Safety is the paramount concern in hydrogenation experiments. The liner material serves as the primary containment vessel against system pressure.
Using 316 L stainless steel provides the necessary compressive strength to operate safely. Based on standard laboratory configurations, this material is reliable for environments reaching up to 20 bar.
Mechanical Robustness
Beyond simple pressure containment, the liner must maintain its shape and integrity under stress.
The superior mechanical properties of this steel grade prevent deformation. This ensures the reactor geometry remains constant, which is vital for consistent flow dynamics and reproducible results.
Understanding the Operational Limits
Pressure Constraints
While 316 L stainless steel is robust, it is not infinite in its capacity.
You must strictly adhere to the tested operational limits, such as the 20 bar threshold. Exceeding this limit compromises the compressive strength benefits and introduces significant safety risks.
Dependency on Design Features
The material provides the potential for success, but the physical design unlocks it.
Without the specific inclusion of a reactor head and thermocouple sleeve, the high thermal conductivity of the material cannot be effectively monitored. You cannot rely on the material properties alone; they must be paired with invasive monitoring to map the reaction accurately.
Making the Right Choice for Your Goal
To maximize the success of your carbon dioxide hydrogenation experiments, align your material selection with your specific operational needs.
- If your primary focus is Operational Safety: Prioritize 316 L stainless steel to ensure superior compressive strength capable of handling pressures up to 20 bar.
- If your primary focus is Thermal Data Accuracy: Ensure your reactor design includes a thermocouple sleeve to leverage the material's conductivity for real-time gradient monitoring.
Select a liner that acts not just as a vessel, but as an active participant in thermal management and safety.
Summary Table:
| Feature | Impact on CO2 Hydrogenation | Material Benefit (316L Stainless Steel) |
|---|---|---|
| Pressure Resistance | Prevents structural failure at 20 bar | High compressive strength ensures safety |
| Thermal Conductivity | Manages reaction heat and prevents hotspots | Efficient heat transfer for thermal stability |
| Data Accuracy | Requires precise temperature monitoring | Allows integration of thermocouple sleeves |
| Mechanical Integrity | Ensures consistent flow and reproducibility | Resists deformation under high stress |
Optimize Your CO2 Hydrogenation Research with KINTEK
Don't let thermal runaway or structural failure compromise your laboratory results. KINTEK provides expert-engineered solution for high-temperature and high-pressure environments. Our laboratory reactors are backed by industry-leading R&D and manufacturing, offering customizable systems—including Muffle, Tube, Rotary, Vacuum, and CVD furnaces—tailored to your specific experimental needs.
Ready to enhance your lab's safety and data precision? Contact KINTEK today to discuss your custom reactor needs
Visual Guide
References
- Albert Gili, Reinhard Schomäcker. One-pot synthesis of iron-doped ceria catalysts for tandem carbon dioxide hydrogenation. DOI: 10.1039/d4cy00439f
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why is a continuous flow of high-purity nitrogen necessary during the pyrolysis of carbon materials in a tube furnace?
- What are the advantages of Quartz Tube Furnaces in terms of customizability and specifications? Unlock Precision and Flexibility for Your Lab
- What temperature is maintained by the water cooling system in Quartz Tube Furnaces? Ensure Seal Integrity at 20°C
- How did the tube furnace originate and where is it commonly used today? Discover Its Evolution and Modern Applications
- How do tube furnaces work? Achieve Precise Thermal Processing for Your Materials
- What are the different heating methods in tube furnaces and their corresponding temperature ranges?
- Why is a vacuum tube furnace required for (Si/graphite/graphene)@C composite? Ensure Optimal High-Temp Performance
- What is the primary function of a Drop Tube Furnace? Master Single-Particle Solid Fuel Ignition Analysis



















