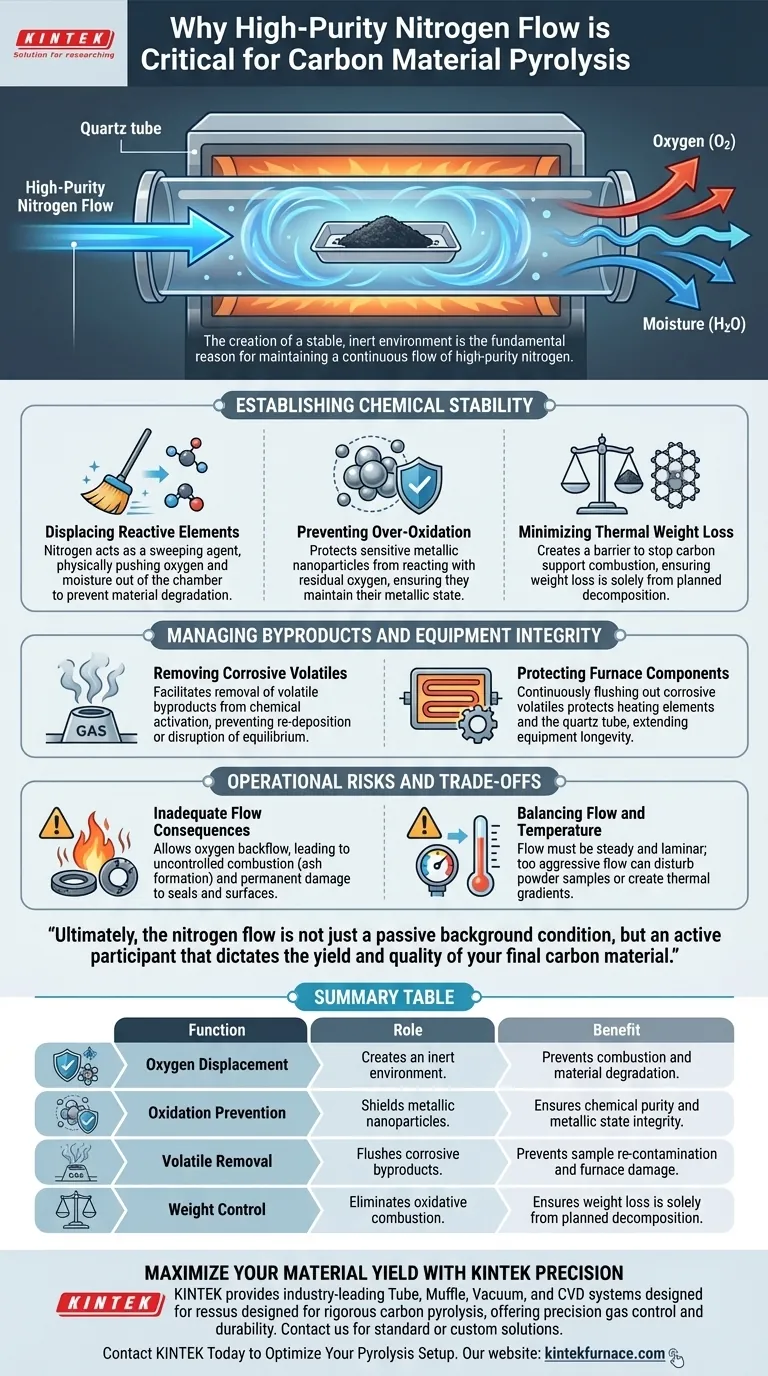
The creation of a stable, inert environment is the fundamental reason for maintaining a continuous flow of high-purity nitrogen. By actively displacing atmospheric oxygen and moisture, the nitrogen stream ensures the reaction remains reductive or neutral rather than oxidative. This protection is essential to prevent the over-oxidation of metallic nanoparticles and to minimize unwanted thermal weight loss in the carbon support during the high-temperature pyrolysis process.
By acting as both a protective shield against combustion and a carrier for volatile byproducts, a continuous nitrogen flow preserves the chemical structure of your material while protecting your equipment from corrosive damage.

Establishing Chemical Stability
Displacing Reactive Elements
The primary threat to pyrolysis is the presence of oxygen and moisture within the furnace chamber. High-purity nitrogen acts as a sweeping agent, physically pushing these reactive elements out of the tube. Without this displacement, the environment would remain oxidative, leading to immediate material degradation.
Preventing Over-Oxidation
For processes involving metallic nanoparticles, the risk of oxidation is acute. A continuous inert flow prevents these sensitive components from reacting with residual oxygen. This ensures the nanoparticles maintain their metallic state rather than converting into unwanted oxides.
Minimizing Thermal Weight Loss
Carbon materials are highly susceptible to combustion at pyrolysis temperatures. Nitrogen creates a barrier that stops the carbon support from burning away ("oxidative combustion"). This ensures that any weight loss is due to the planned decomposition of the precursor, not the destruction of the carbon structure itself.
Managing Byproducts and Equipment Integrity
Removing Corrosive Volatiles
During chemical activation (often using agents like KOH or ZnCl2), the reaction generates significant volatile byproducts. A precision nitrogen flow facilitates the removal of these gases. If these volatiles were allowed to stagnate, they could disrupt the chemical equilibrium of the reaction or re-deposit onto the sample.
Protecting Furnace Components
The byproducts of pyrolysis and activation can be highly corrosive to the internal components of the furnace. By continuously flushing these corrosive volatiles out of the chamber, the nitrogen stream protects the equipment. This is critical for maintaining the longevity of the heating elements and the quartz tube itself.
Operational Risks and Trade-offs
The Consequence of Inadequate Flow
Failing to maintain a sufficient continuous flow allows oxygen to diffuse back into the chamber or volatiles to accumulate. This leads to uncontrolled combustion, resulting in ash rather than activated carbon. Furthermore, stagnant corrosive gases can permanently damage the tube furnace seals and internal surfaces.
Balancing Flow and Temperature
While flow is essential, it must be carefully controlled. A flow that is too aggressive can disturb lightweight powder samples or create thermal gradients. The goal is a steady, laminar flow that clears the atmosphere without physically displacing the sample material.
Making the Right Choice for Your Goal
To optimize your pyrolysis process, align your nitrogen flow strategy with your specific objectives:
- If your primary focus is Material Purity: Ensure the nitrogen is high-purity to strictly prevent the over-oxidation of metallic nanoparticles and preserve the carbon support structure.
- If your primary focus is Equipment Longevity: Prioritize a consistent flow rate high enough to rapidly evacuate corrosive volatiles generated by activation agents like KOH.
Ultimately, the nitrogen flow is not just a passive background condition, but an active participant that dictates the yield and quality of your final carbon material.
Summary Table:
| Function | Role in Pyrolysis | Benefit to Sample/Equipment |
|---|---|---|
| Oxygen Displacement | Creates an inert environment | Prevents combustion and material degradation |
| Oxidation Prevention | Shields metallic nanoparticles | Ensures chemical purity and metallic state integrity |
| Volatile Removal | Flushes corrosive byproducts | Prevents sample re-contamination and furnace damage |
| Weight Control | Eliminates oxidative combustion | Ensures weight loss is solely from planned decomposition |
Maximize Your Material Yield with KINTEK Precision
Don't let oxidation or corrosive volatiles compromise your research. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems designed to handle the rigorous demands of carbon pyrolysis. Backed by expert R&D and manufacturing, our high-temperature furnaces offer the precision gas control and durability your lab requires. Whether you need a standard setup or a fully customizable solution for unique thermal profiles, our experts are here to help.
Contact KINTEK Today to Optimize Your Pyrolysis Setup
Visual Guide
References
- Lilian Moumaneix, Tanja Kallio. Zero‐Valent Iron Nanoparticles Supported on Si/N Codoped Carbon Materials: From Biomass to Oxygen Reduction Electrocatalysts and Supercapacitors. DOI: 10.1002/aesr.202500092
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the function of a fast-response photoelectric sensor system? Precision Ignition Timing in Tube Furnaces
- What are the common applications of tube furnaces in laboratories? Discover Versatile High-Temperature Solutions
- What is the primary role of a high-temperature tube furnace in Ga2O3 annealing? Optimize Your Thin Film Quality
- Why is pre-oxidation treatment of the substrate in a tube furnace necessary? Ensure Strong Ti(Nb)-Si-C Coating Adhesion
- What recent advancements have enhanced the performance of lab tubular furnaces? Achieve Unprecedented Precision & Control
- Why is alumina ceramic tubing selected as the liner for a Drop Tube Furnace? Ensure Purity and High-Temp Stability
- What is the core role of a tube furnace in synthesizing magnetic carbon-based composites? Expert Insights
- Why are high-temperature tube furnaces essential for perovskite catalysts? Precision Shaping & Crystallization



















