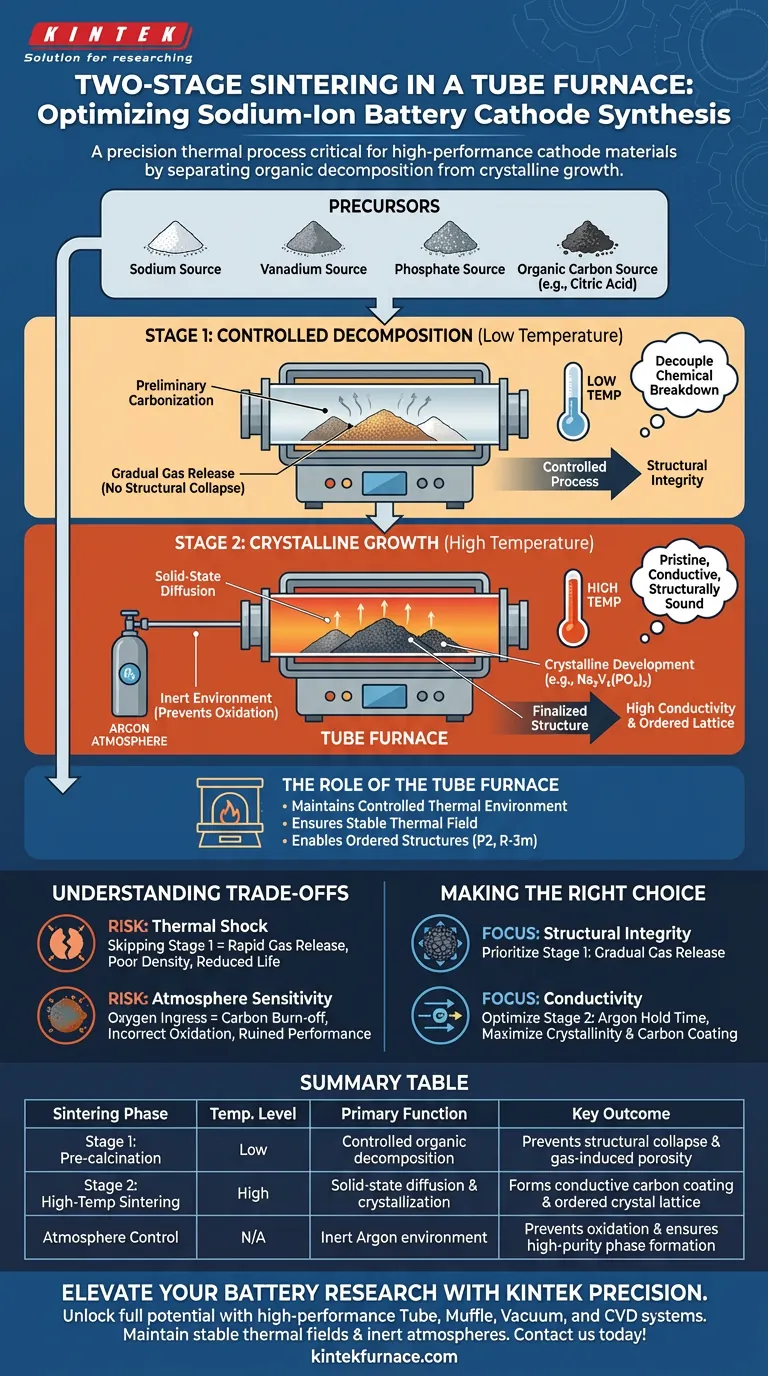
A two-stage sintering process is critical for synthesizing high-performance sodium-ion battery cathodes because it separates organic decomposition from crystalline growth. By utilizing a tube furnace to execute a low-temperature pre-calcination followed by high-temperature sintering, you prevent structural collapse caused by rapid gas release while ensuring the formation of a highly conductive carbon coating.
Core Takeaway Achieving high-performance cathode materials requires decoupling the chemical breakdown of precursors from the final structural ordering. The two-stage process allows for the gentle removal of volatile organics first, ensuring that the subsequent high-temperature phase yields a pristine, conductive, and structurally sound crystal lattice.
The Logic Behind the Two-Stage Approach
The synthesis of complex materials like sodium vanadium phosphate requires precision. A tube furnace provides the stable thermal field necessary to execute this in two distinct phases, each serving a specific structural function.
Stage 1: Controlled Decomposition (Low Temperature)
The first stage is a pre-calcination step. Its primary purpose is the controlled breakdown of organic components, such as citric acid, which are often used as carbon sources or chelating agents.
During this phase, the tube furnace operates at a lower temperature to facilitate preliminary carbonization.
This step is vital for structural integrity. If the material were immediately subjected to high heat, the organic components would decompose violently. This rapid gas evolution would cause the material structure to collapse or become porous in an uncontrolled manner.
Stage 2: Crystalline Growth (High Temperature)
Once the volatile organics are safely decomposed, the process moves to the high-temperature sintering stage. This is typically conducted under an inert argon atmosphere within the tube furnace.
This stage drives the solid-state diffusion reactions necessary for performance. It promotes the full crystalline development of materials like sodium vanadium phosphate and sodium vanadium phosphate fluorophosphate.
Simultaneously, this high heat finalizes the formation of an in-situ carbon layer. This uniform coating acts as a conductive network, which is essential for the electronic conductivity of the final battery cathode.
The Role of the Tube Furnace
The tube furnace is the enabler of this entire process. It maintains a controlled thermal environment, which is essential for solid-state reactions.
Whether synthesizing layered oxides or phosphates, the furnace ensures the thermal field is stable over extended periods. This stability allows constituent elements to arrange into ordered structures (such as P2 or R-3m space groups), resulting in high purity and high crystallinity.
Understanding the Trade-offs
While the two-stage process is superior for performance, it requires careful management of process parameters.
The Risk of Thermal Shock
Skipping the low-temperature stage effectively "shocks" the material. Without pre-calcination, the rapid release of gases destroys the particle morphology, leading to poor density and reduced battery life.
Atmosphere Sensitivity
The high-temperature stage relies heavily on the atmosphere. For sodium vanadium phosphate, an inert argon environment is non-negotiable.
If the tube furnace atmosphere is compromised (e.g., accidental oxygen ingress), the conductive carbon layer may burn off, or the transition metal (Vanadium) may oxidize incorrectly, ruining the electrochemical performance.
Making the Right Choice for Your Goal
To maximize the potential of your sodium-ion cathode materials, align your sintering protocol with your specific performance targets.
- If your primary focus is Structural Integrity: Prioritize the low-temperature pre-calcination stage to ensure gradual gas release and prevent particle collapse.
- If your primary focus is Conductivity: Optimize the high-temperature argon hold time to maximize the crystallinity of the active material and the uniformity of the carbon coating.
Success in synthesis comes from respecting that decomposition and crystallization are distinct processes that require different thermal environments.
Summary Table:
| Sintering Phase | Temperature Level | Primary Function | Key Outcome |
|---|---|---|---|
| Stage 1: Pre-calcination | Low | Controlled organic decomposition | Prevents structural collapse & gas-induced porosity |
| Stage 2: High-Temp Sintering | High | Solid-state diffusion & crystallization | Forms conductive carbon coating & ordered crystal lattice |
| Atmosphere Control | N/A | Inert Argon environment | Prevents oxidation & ensures high-purity phase formation |
Elevate Your Battery Research with KINTEK Precision
Unlock the full potential of your sodium-ion cathode materials with KINTEK’s industry-leading thermal solutions. Backed by expert R&D and precision manufacturing, KINTEK offers high-performance Tube, Muffle, Vacuum, and CVD systems designed to maintain the stable thermal fields and inert atmospheres required for complex two-stage sintering.
Whether you are refining layered oxides or advanced phosphates, our customizable lab furnaces provide the control you need for superior crystallinity and conductivity. Contact us today to find the perfect furnace for your unique synthesis needs!
Visual Guide
References
- Yi Yang, He-Zhang Chen. Na <sub>3</sub> V <sub>2</sub> (PO <sub>4</sub> ) <sub>3</sub> -decorated Na <sub>3</sub> V <sub>2</sub> (PO <sub>4</sub> ) <sub>2</sub> F <sub>3</sub> as a high-rate and cycle-stable cathode material for sodium ion batteries. DOI: 10.1039/d4ra01653j
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the function of a fast-response photoelectric sensor system? Precision Ignition Timing in Tube Furnaces
- What is the technical significance of a horizontal tube furnace with a sliding rail for NiOx annealing? Enhance Control
- What function does a tube furnace perform in sintering boron duplex stainless steel? Master High-Precision Results
- What types of atmospheres can be controlled in a drop tube furnace? Master Precise Gas Control for Superior Materials
- Why is a high-temperature tube furnace required for Ti3AuC2 annealing? Achieve Perfect Atomic Exchange
- Why is a nitrogen flow control system necessary for a tube furnace? Prevent Oxidation and Ensure Carbonization Yield
- What is the role of a vacuum tube furnace during the final thermal treatment stage of Fe3O4@CSAC catalysts?
- In which fields are fluidized bed vertical tube furnaces commonly applied? Explore Key Uses in Materials Science and Energy



















