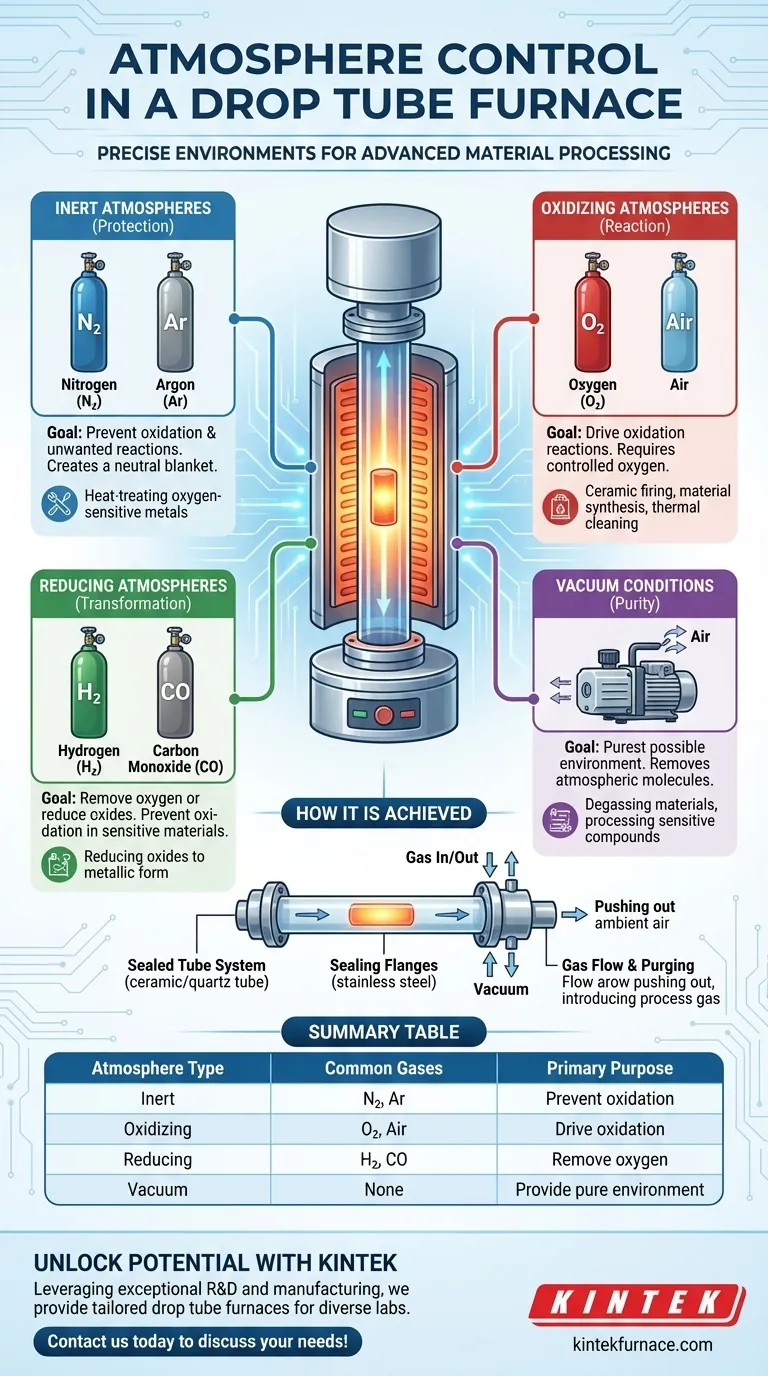
At its core, a drop tube furnace is designed for precise atmospheric control. It can successfully operate under inert, oxidizing, and reducing gas atmospheres, as well as under vacuum conditions, to meet the specific requirements of your material processing.
A drop tube furnace’s primary advantage over other furnace types is its ability to create a highly controlled, isolated environment. By sealing the process tube and introducing specific gases, you can prevent unwanted chemical reactions like oxidation or intentionally drive desired ones, directly influencing your material's final properties.

How Atmosphere Control is Achieved
The ability to manipulate the atmosphere inside a drop tube furnace is not accidental; it is a key design feature. This control is fundamental to achieving reproducible, high-quality results in material synthesis and heat treatment.
The Sealed Tube System
A tube furnace uses a ceramic or quartz tube to contain the sample. Unlike a muffle furnace that heats in open air, this tube can be completely sealed off from the outside environment.
The Role of Sealing Flanges
Specialized sealing flanges, typically made of stainless steel, are attached to the ends of the tube. These flanges contain ports for gas inlets, gas outlets, and vacuum pumps, creating a closed-loop system that ensures the internal atmosphere remains pure.
Gas Flow and Purging
To establish a specific atmosphere, the system is first purged. An inert gas is flowed through the tube to push out the ambient air. Once purged, the desired process gas (inert, reactive, or a mixture) is introduced at a controlled flow rate to maintain the environment throughout the heating cycle.
A Breakdown of Common Atmospheres and Their Purpose
The choice of atmosphere is dictated entirely by your process goal. Each category serves a distinct chemical purpose.
Inert Atmospheres (Protection)
Inert gases like Nitrogen (N₂) and Argon (Ar) are used when the goal is to heat a material without it reacting with its surroundings. They create a neutral blanket, preventing oxidation and other unwanted chemical changes. This is critical for heat-treating oxygen-sensitive metals and alloys.
Oxidizing Atmospheres (Reaction)
An oxidizing atmosphere is created by introducing gases like Oxygen (O₂) or air. This environment is used when the process requires oxidation. Applications include certain types of ceramic firing, material synthesis, or thermal cleaning processes where organic binders must be burned off.
Reducing Atmospheres (Transformation)
Reducing atmospheres use reactive gases like Hydrogen (H₂) or Carbon Monoxide (CO). Their purpose is to remove oxygen from a material (i.e., to "reduce" it). This is essential for preventing oxidation in highly sensitive materials or for specific chemical reactions where oxides need to be converted back into their metallic form.
Vacuum Conditions (Purity)
For the highest level of protection, the furnace tube can be evacuated using a vacuum pump. Operating under vacuum removes virtually all atmospheric molecules, providing the purest possible environment. This is ideal for degassing materials or processing extremely sensitive compounds that could react with even trace amounts of gas.
Understanding the Trade-offs and Limitations
While powerful, atmosphere control is not without its challenges. Success depends on careful setup and an awareness of potential pitfalls.
Sealing Integrity is Critical
The effectiveness of your atmosphere control is only as good as your seals. Any leak in the sealing flanges or connections will allow ambient air to contaminate the process, compromising your results. Regular inspection and maintenance are essential.
Gas Purity and Flow Control
The purity of your source gas directly impacts the purity of the furnace atmosphere. Similarly, inconsistent flow rates can lead to pressure fluctuations and an unstable environment. Using high-purity gases and a reliable mass flow controller is key to reproducibility.
Safety with Reactive Gases
Using flammable or toxic gases like Hydrogen (H₂) and Carbon Monoxide (CO) introduces significant safety risks. These processes demand robust safety protocols, proper ventilation, and gas detection systems to prevent accidents.
Selecting the Right Atmosphere for Your Process
Your choice of atmosphere should be a direct reflection of your desired outcome. Consider the goal of your heat treatment to make an informed decision.
- If your primary focus is preventing oxidation or unwanted reactions: Use an inert gas like Argon or Nitrogen, or for maximum purity, operate under vacuum.
- If your primary focus is to drive an oxidation reaction: Use a controlled flow of Oxygen or clean, dry air.
- If your primary focus is removing oxygen or transforming an oxide: Use a reducing atmosphere containing Hydrogen or Carbon Monoxide, with all necessary safety precautions.
- If your primary focus is degassing or processing highly sensitive materials: Use a vacuum to create the purest possible environment.
Ultimately, mastering atmosphere control in a drop tube furnace gives you direct command over the chemical and physical properties of your final material.
Summary Table:
| Atmosphere Type | Common Gases | Primary Purpose |
|---|---|---|
| Inert | Nitrogen (N₂), Argon (Ar) | Prevent oxidation and unwanted reactions |
| Oxidizing | Oxygen (O₂), Air | Drive oxidation reactions |
| Reducing | Hydrogen (H₂), Carbon Monoxide (CO) | Remove oxygen or reduce oxides |
| Vacuum | None (evacuated) | Provide pure environment for sensitive processes |
Unlock the full potential of your material processing with KINTEK's advanced high-temperature furnace solutions. Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with tailored drop tube furnaces, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs, enhancing efficiency and results. Contact us today to discuss how we can support your specific atmosphere control requirements and drive innovation in your lab!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What does inert mean in furnace atmospheres? Protect materials from oxidation with inert gases.
- How does an inert atmosphere prevent oxidation? Shield Materials from Oxygen Damage
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality



















