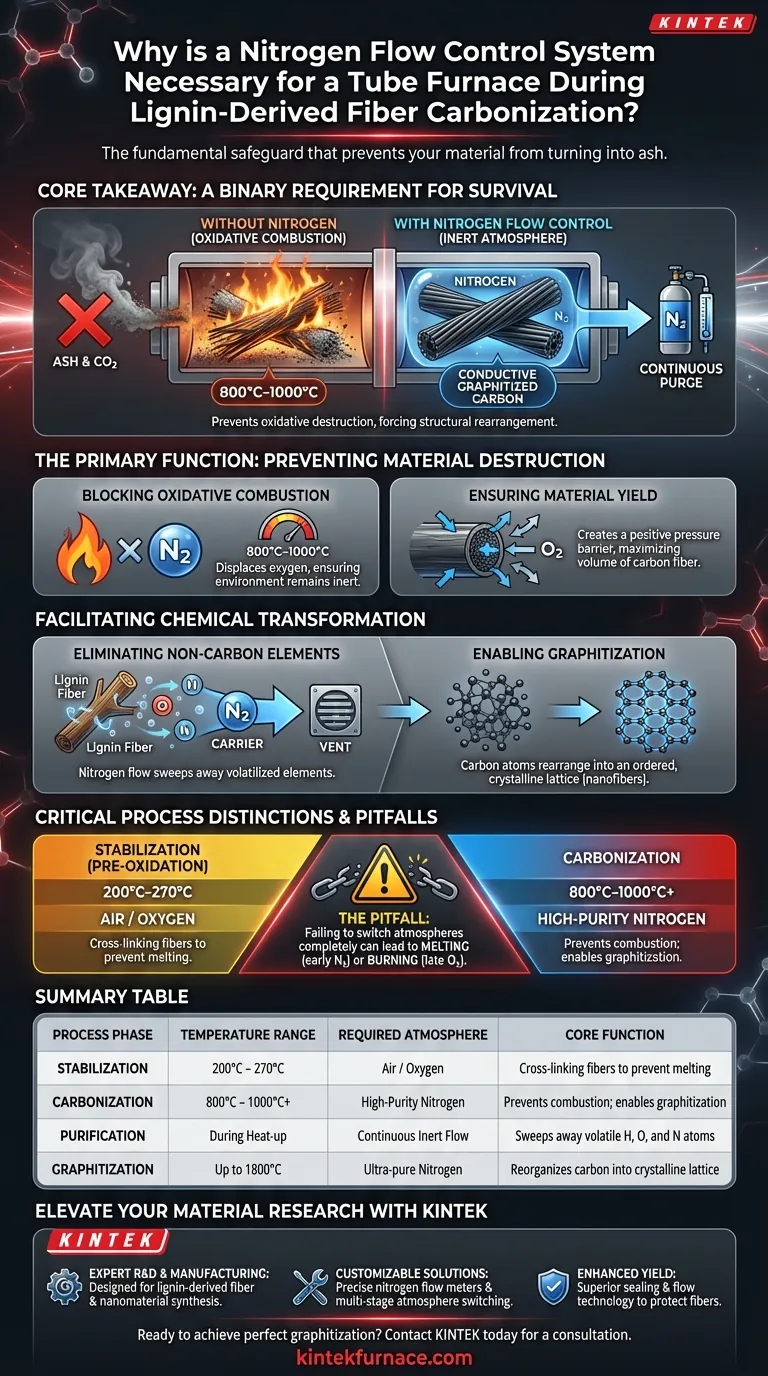
A nitrogen flow control system is the fundamental safeguard that prevents your material from turning into ash. During the carbonization of lignin-derived fibers, this system maintains a strictly inert atmosphere, blocking oxygen from entering the furnace chamber. Without this continuous purge of nitrogen, the high processing temperatures would cause the fibers to undergo oxidative combustion—burning them away completely rather than converting them into carbon.
Core Takeaway A nitrogen environment is not merely for optimization; it is a binary requirement for survival of the material. It prevents the oxidative destruction of the fiber at high temperatures (800°C–1000°C), forcing the material to shed non-carbon atoms and structurally rearrange into conductive, graphitized carbon nanofibers.
The Primary Function: Preventing Material Destruction
Blocking Oxidative Combustion
Carbonization occurs at aggressive temperatures, typically between 800 °C and 1000 °C. At these heat levels, carbon is highly reactive with oxygen.
If the furnace atmosphere contained standard air, the lignin fibers would simply burn. The nitrogen flow displaces oxygen, ensuring the environment remains inert. This preserves the physical structure of the fiber, preventing it from turning into ash or carbon dioxide.
Ensuring Material Yield
The efficiency of the process is measured by the yield of the final product. Even trace amounts of oxygen can lead to partial "ashing," where outer layers of the fiber are consumed.
A continuous, controlled nitrogen flow creates a positive pressure barrier. This prevents outside air from leaking in and ensures the carbon framework remains intact, maximizing the volume of activated carbon or carbon fiber produced.
Facilitating Chemical Transformation
Eliminating Non-Carbon Elements
The goal of carbonization is to purify the material. The heat drives out non-carbon elements found in the lignin precursor, specifically nitrogen, oxygen, and hydrogen.
The nitrogen flow acts as a carrier mechanism. As these elements volatilize (turn into gas), the flowing nitrogen sweeps them out of the hot zone. This prevents them from redepositing on the fibers or interfering with the purity of the carbon structure.
Enabling Graphitization
Once the non-carbon elements are removed, the remaining carbon atoms must reorganize. This process is called graphitization.
In the protected nitrogen atmosphere, carbon atoms undergo a structural rearrangement. They shift from a chaotic, amorphous state into an ordered, crystalline lattice. This transformation is what gives the final nanofibers their high electrical conductivity and superior thermal stability.
Critical Process Distinctions and Pitfalls
The Danger of Wrong Atmospheres
It is vital to distinguish between carbonization and stabilization.
- Stabilization (Pre-oxidation): This occurs at lower temperatures (200–270°C) and actually requires an air atmosphere to cross-link the fibers so they don't melt.
- Carbonization: This occurs at high temperatures (800°C+) and requires nitrogen.
A common pitfall is failing to switch atmospheres completely. If nitrogen is introduced too early (during stabilization), the fibers may melt. If oxygen remains during carbonization, the fibers will burn.
Temperature Control and Purity
The uniformity of the thermal field affects the quality of the graphite structure.
While the nitrogen prevents burning, the temperature profile (heating rate and dwell time) drives the density of the fiber. The system must maintain inertness all the way up to extreme temperatures (sometimes reaching 1800°C for advanced applications) to achieve the highest order of turbostratic graphite structure.
Making the Right Choice for Your Goal
To ensure the success of your lignin-fiber project, apply the nitrogen control based on your specific endpoint:
- If your primary focus is High Conductivity: Ensure your system can maintain a pure nitrogen flow at temperatures exceeding 1000°C to maximize graphitization and atomic ordering.
- If your primary focus is Fiber Integrity (Yield): Prioritize a system with precise flow control to prevent turbulence or oxygen leaks that could cause surface ashing and reduce material recovery.
- If your primary focus is Process Safety: Verify that the system can switch distinctively from an air atmosphere (for stabilization) to a nitrogen atmosphere (for carbonization) without cross-contamination.
Ultimately, the nitrogen flow control system transforms your furnace from a simple incinerator into a precision reactor capable of creating advanced nanomaterials.
Summary Table:
| Process Phase | Temperature Range | Required Atmosphere | Core Function |
|---|---|---|---|
| Stabilization | 200°C – 270°C | Air / Oxygen | Cross-linking fibers to prevent melting |
| Carbonization | 800°C – 1000°C+ | High-Purity Nitrogen | Prevents combustion; enables graphitization |
| Purification | During Heat-up | Continuous Inert Flow | Sweeps away volatile H, O, and N atoms |
| Graphitization | Up to 1800°C | Ultra-pure Nitrogen | Reorganizes carbon into crystalline lattice |
Elevate Your Material Research with KINTEK
Don't let your research turn to ash. Precision carbonization requires uncompromising atmosphere control. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems specifically engineered for advanced thermal processing.
Why partner with us?
- Expert R&D & Manufacturing: Our systems are designed for the rigorous demands of lignin-derived fiber and nanomaterial synthesis.
- Customizable Solutions: Whether you need precise nitrogen flow meters or multi-stage atmosphere switching, we tailor our high-temp furnaces to your unique specs.
- Enhanced Yield: Protect your fibers from oxidative destruction with our superior sealing and flow technology.
Ready to achieve perfect graphitization? Contact KINTEK today for a consultation and let our experts help you build the ideal thermal environment.
Visual Guide
References
- Meruyert Nazhipkyzy, Dana D. Assylkhanova. Synthesis of Lignin/PAN Fibers from Sawdust. DOI: 10.3390/fib12030027
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- How do sealed flanges improve oxygen annealing for superconducting joints? Enhance Purity and Precision
- What are the disadvantages of tube furnace cracking when processing heavy raw materials? Avoid Costly Downtime and Inefficiency
- How does a high-temperature tube furnace form Nitrogen-doped Porous Carbon (RMF)? Precision Thermal Synthesis Guide
- What role does a high-temperature tube furnace play in the post-processing of high-entropy alloys? Optimize Microstructure
- What is the primary function of controlled thermal processing for YIG thin films? Unlock Magnetic Order in Spintronics
- How does the environmental control within a high-temperature tube furnace affect Ag-N-C catalyst pyrolysis?
- What role does a vacuum tube furnace play in the 600°C high-temperature annealing of Pd/TaTiNbZr/Ta multilayer membranes?
- What is the function of a vacuum tube furnace in the regeneration of expanded graphite? Deep Pore Restoration Expert



















