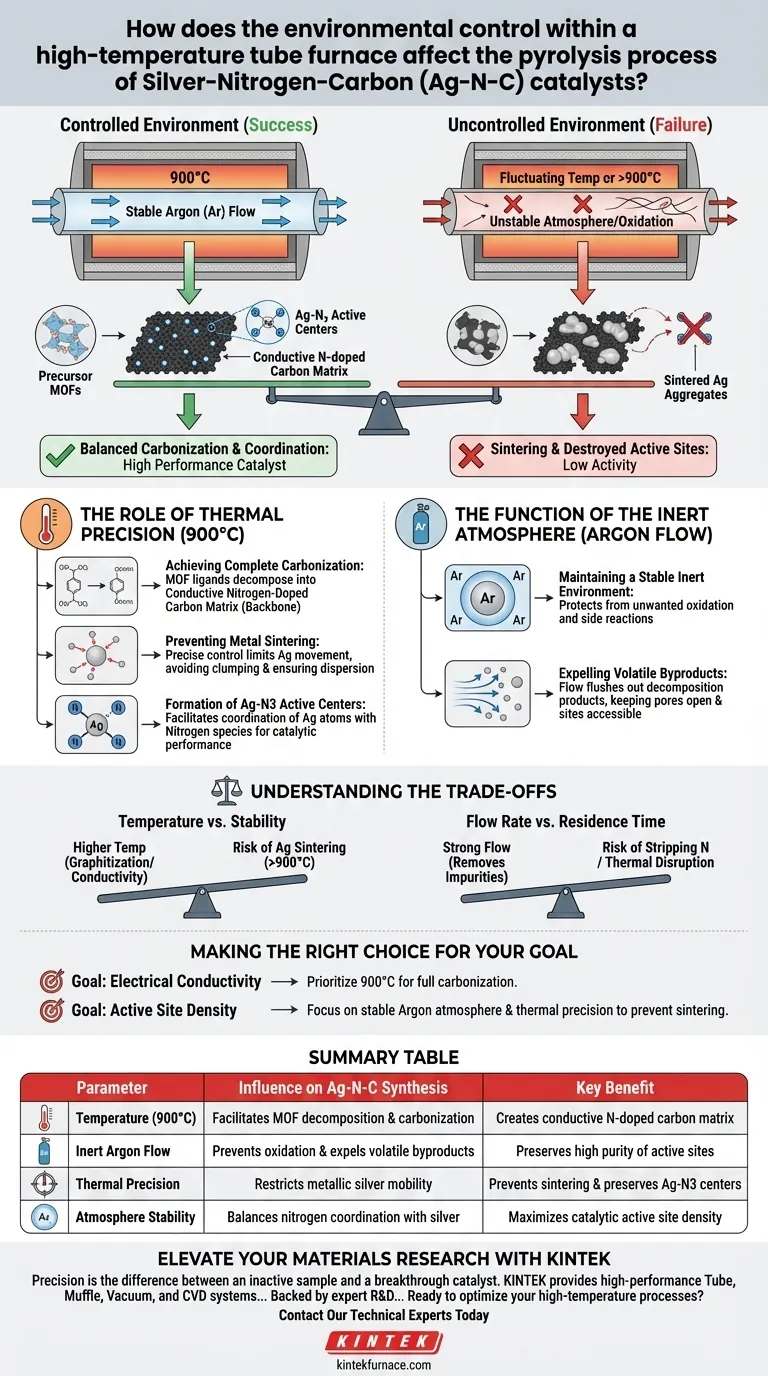
The precise environmental control within a high-temperature tube furnace determines the structural integrity and electrochemical performance of Silver-Nitrogen-Carbon (Ag-N-C) catalysts. Specifically, maintaining a strict temperature gradient at 900°C under a stable Argon (Ar) atmosphere is required to convert metal-organic frameworks (MOFs) into a conductive nitrogen-doped carbon matrix while simultaneously preventing silver agglomeration.
Core Insight: The tube furnace does not simply heat the material; it orchestrates a delicate balance between carbonization (ensuring conductivity) and coordination (stabilizing active sites). Without this controlled environment, the silver particles would sinter, destroying the specific Ag-N3 active centers required for catalytic activity.

The Role of Thermal Precision
The transformation of precursor materials into a functional catalyst relies heavily on the specific thermal profile applied during pyrolysis.
Achieving Complete Carbonization
The tube furnace must provide a precise temperature gradient, reaching 900°C.
At this temperature, the organic ligands within the Metal-Organic Frameworks (MOFs) undergo complete decomposition.
This process converts the organic material into a conductive nitrogen-doped carbon matrix, which serves as the physical backbone of the catalyst.
Preventing Metal Sintering
One of the greatest risks during high-temperature pyrolysis is the aggregation of metal particles.
Precise thermal control limits the movement of metallic silver, preventing the particles from clumping together (sintering).
By avoiding sintering, the furnace ensures the silver remains atomically dispersed or forms small, stable nanoclusters rather than large, inactive chunks.
Formation of Ag-N3 Active Centers
The interaction between the silver and the nitrogen-doped carbon happens at a molecular level.
The specific thermal environment at 900°C facilitates the coordination of silver atoms with nitrogen species.
This results in the formation of stable Ag-N3 active centers, which are the specific chemical sites responsible for the catalyst's performance.
The Function of the Inert Atmosphere
Thermal energy alone is insufficient; the chemical atmosphere within the tube affects how the precursor decomposes and re-forms.
Maintaining a Stable Inert Environment
The primary reference highlights the necessity of using an Argon (Ar) flow.
This creates a stable, inert atmosphere that protects the catalyst from unwanted oxidation or side reactions during the critical heating phase.
Expelling Volatile Byproducts
While the primary mechanism is protection, the continuous flow of gas plays a secondary role in purity.
The flow helps expel volatile decomposition products generated during the breakdown of organic ligands.
Removing these byproducts prevents them from re-depositing on the catalyst surface, ensuring the pores remain open and the active sites accessible.
Understanding the Trade-offs
When configuring a tube furnace for Ag-N-C synthesis, you must navigate specific operational trade-offs to avoid failure.
Temperature vs. Stability
Raising the temperature promotes better graphitization and conductivity of the carbon support.
However, exceeding the optimal 900°C threshold significantly increases the risk of silver sintering, which destroys the Ag-N3 active centers.
Flow Rate vs. Residence Time
A strong gas flow effectively removes impurities and protects the sample.
However, if the flow is too aggressive, it may disrupt the local thermal equilibrium or strip away nitrogen species before they can coordinate with the silver.
Making the Right Choice for Your Goal
To maximize the performance of your Ag-N-C catalyst, you must tailor the furnace controls to your specific objective.
- If your primary focus is Electrical Conductivity: Prioritize maintaining the 900°C temperature to ensure the organic ligands are fully converted into a graphitized carbon matrix.
- If your primary focus is Active Site Density: Focus on the stability of the Argon atmosphere and thermal precision to prevent sintering and preserve the delicate Ag-N3 structures.
Success in Ag-N-C synthesis relies on using the tube furnace to lock silver atoms into a nitrogen-doped lattice without allowing them to fuse together.
Summary Table:
| Parameter | Influence on Ag-N-C Synthesis | Key Benefit |
|---|---|---|
| Temperature (900°C) | Facilitates MOF decomposition & carbonization | Creates conductive N-doped carbon matrix |
| Inert Argon Flow | Prevents oxidation & expels volatile byproducts | Preserves high purity of active sites |
| Thermal Precision | Restricts metallic silver mobility | Prevents sintering & preserves Ag-N3 centers |
| Atmosphere Stability | Balances nitrogen coordination with silver | Maximizes catalytic active site density |
Elevate Your Materials Research with KINTEK
Precision is the difference between an inactive sample and a breakthrough catalyst. KINTEK provides high-performance Tube, Muffle, Vacuum, and CVD systems engineered for the rigorous demands of MOF pyrolysis and Ag-N-C synthesis. Backed by expert R&D and manufacturing, our furnaces ensure the thermal stability and atmosphere control required to prevent sintering and preserve delicate active sites.
Ready to optimize your high-temperature processes? Our systems are fully customizable to meet your unique laboratory needs.
Contact Our Technical Experts Today
Visual Guide
References
- M. Nur Hossain, Gianluigi A. Botton. Efficient Electrochemical CO<sub>2</sub> Reduction Using AgN<sub>3</sub> Single‐Atom Sites Embedded in Free‐Standing Electrodes for Flow Cell Applications. DOI: 10.1002/smsc.202400643
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How does a laboratory high-temperature tube furnace contribute to the conversion of electrospun fibers? Expert Insights
- What role does a tube sintering furnace play in the calcination of Lithium Iron Phosphate? Optimizing LFP Performance
- Why is precise heating rate control in a high-temperature tube furnace critical for HyDR? Master Reduction Kinetics
- How does a vacuum tube furnace function in Ti6Al4V post-processing? Optimize Additive Manufacturing Outcomes
- How does a vertical tube furnace comply with environmental standards? Achieve Eco-Friendly and Efficient Lab Operations
- Why is uniform heating important in tubular furnaces? Ensure Process Reliability and Predictable Results
- What are the space-saving benefits of a tube furnace? Maximize Lab Efficiency with Compact Design
- What is the significance of the 700°C tube furnace treatment for T-Nb2O5/RMF? Unlock Peak Pseudocapacitive Performance



















