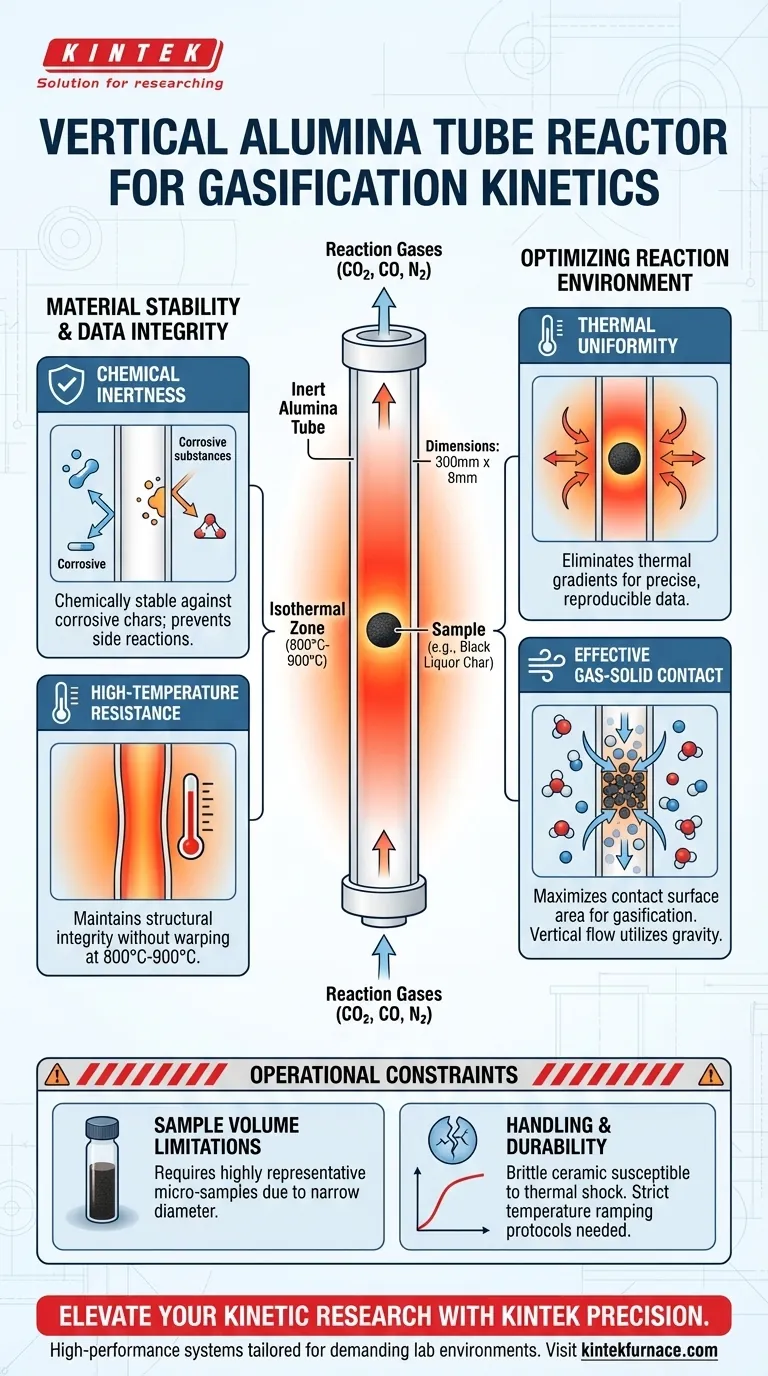
A vertical alumina tube reactor functions as the primary, high-precision vessel for isolating gas-solid interactions during gasification kinetics research. By positioning the sample in the center of the tube, typically within an 800°C to 900°C isothermal zone, it ensures the material is heated uniformly while allowing reaction gases like CO2, CO, and N2 to flow vertically through the sample. This specific configuration is critical for eliminating environmental variables, allowing researchers to measure reaction rates and carbon conversion without interference.
The reactor's vertical design and inert alumina construction provide a stable, chemically neutral environment that guarantees kinetic data is derived solely from the gasification process, unaffected by thermal gradients or reactor wall interactions.
Optimizing the Reaction Environment
To understand the utility of this reactor, one must look at how it controls the physical environment surrounding the sample.
Ensuring Thermal Uniformity
The geometry of the reactor—specifically cited as 300mm in length and 8mm in diameter—is engineered to create a precise "isothermal zone."
By placing the sample in the exact center of this vertical tube, researchers ensure the fuel is exposed to a constant, uniform temperature. This eliminates thermal gradients, which are a common source of error in kinetic modeling.
Facilitating Effective Gas-Solid Contact
The vertical orientation is not arbitrary; it utilizes gravity and flow dynamics to force interaction.
Reaction gases are directed to flow vertically through the tube. Because the sample is constrained within the narrow diameter, the gas molecules are forced to pass through or over the solid sample, maximizing the contact surface area required for gasification.
Material Stability and Data Integrity
Beyond geometry, the material composition of the reactor plays a vital role in the validity of the data collected.
Chemical Inertness
In experiments involving complex fuels, such as black liquor char, the sample can be highly corrosive.
Alumina is selected because it is chemically stable and does not interact with these corrosive chars or the gasifying agents. This ensures that the mass loss or gas evolution measured is purely from the sample, not a side reaction with the reactor walls.
High-Temperature Resistance
Gasification kinetics studies demand high thermal loads, typically in the range of 800°C to 900°C.
The alumina construction maintains structural integrity at these temperatures. It provides a rigid, reliable boundary that contains the reaction without warping or degrading over repeated heating cycles.
Understanding Operational Constraints
While the vertical alumina tube reactor is highly effective, it introduces specific constraints that must be managed to ensure accurate results.
Sample Volume Limitations
The narrow 8mm diameter restricts the quantity of the sample that can be tested at one time.
This requires researchers to use highly representative micro-samples. If the sample is not homogeneous, the small volume may not accurately reflect the behavior of the bulk material.
Handling and Durability
Alumina is a ceramic; while it is thermally stable, it is also brittle.
Unlike metal reactors, it is susceptible to thermal shock if heated or cooled too rapidly. Strict temperature ramping protocols must be followed to prevent the tube from cracking during experiments.
Making the Right Choice for Your Research
When designing your experimental setup, consider how the reactor's specific attributes align with your data requirements.
- If your primary focus is kinetic precision: Prioritize the positioning of the sample within the center of the tube to ensure it sits fully within the isothermal zone.
- If your primary focus is analyzing corrosive feedstocks: Rely on the chemical inertness of the alumina to prevent reaction artifacts that could skew carbon conversion data.
By leveraging the vertical orientation for flow control and the alumina construction for stability, you ensure that your kinetic data reflects the true chemistry of gasification.
Summary Table:
| Function | Benefit to Kinetics Research |
|---|---|
| Vertical Flow Design | Maximizes gas-solid contact and ensures uniform interaction dynamics. |
| Alumina Composition | Provides chemical inertness against corrosive chars and high thermal stability. |
| Isothermal Zone | Eliminates thermal gradients for highly accurate, reproducible reaction rates. |
| Inert Environment | Guarantees kinetic data is derived solely from the process, not the reactor walls. |
Elevate Your Kinetic Research with KINTEK Precision
Are you looking to eliminate variables in your thermal experiments? Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for the most demanding lab environments. Our customizable high-temperature furnaces and alumina reactor solutions provide the stability and precision required for advanced materials science and gasification studies.
Maximize your laboratory efficiency today. Contact our specialists now to discuss your unique project requirements and discover the KINTEK advantage.
Visual Guide
References
- F. Bueno, José Luis Sánchez. CO₂ Gasification of Black Liquor Char under isothermal and dynamic conditions. DOI: 10.26754/jji-i3a.202512008
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What role does the high-temperature vacuum tube furnace play in SiC/SiC pyrolysis? Essential Chemical Transformation
- Why is a tube furnace with an argon atmosphere required for zeolite stabilization? Protect Your Material Structure
- What is a horizontal electric furnace designed for? Achieve Precise Thermal Processing in Controlled Environments
- What functions does a tube atmosphere furnace perform for high-entropy alloy catalysts? Essential Reduction & Control
- What is the specific purpose of using a laboratory tube furnace with a wet argon environment? Optimize Siloxane Curing
- How does high-temperature tube furnace programmed control influence porous carbon? Expert Pore Geometry Insights
- What are the main benefits of using a tube furnace? Achieve Precise Temperature and Atmosphere Control
- What role does a tube furnace play in the chemical activation of eucalyptus biochar? Precision Thermal Activation



















