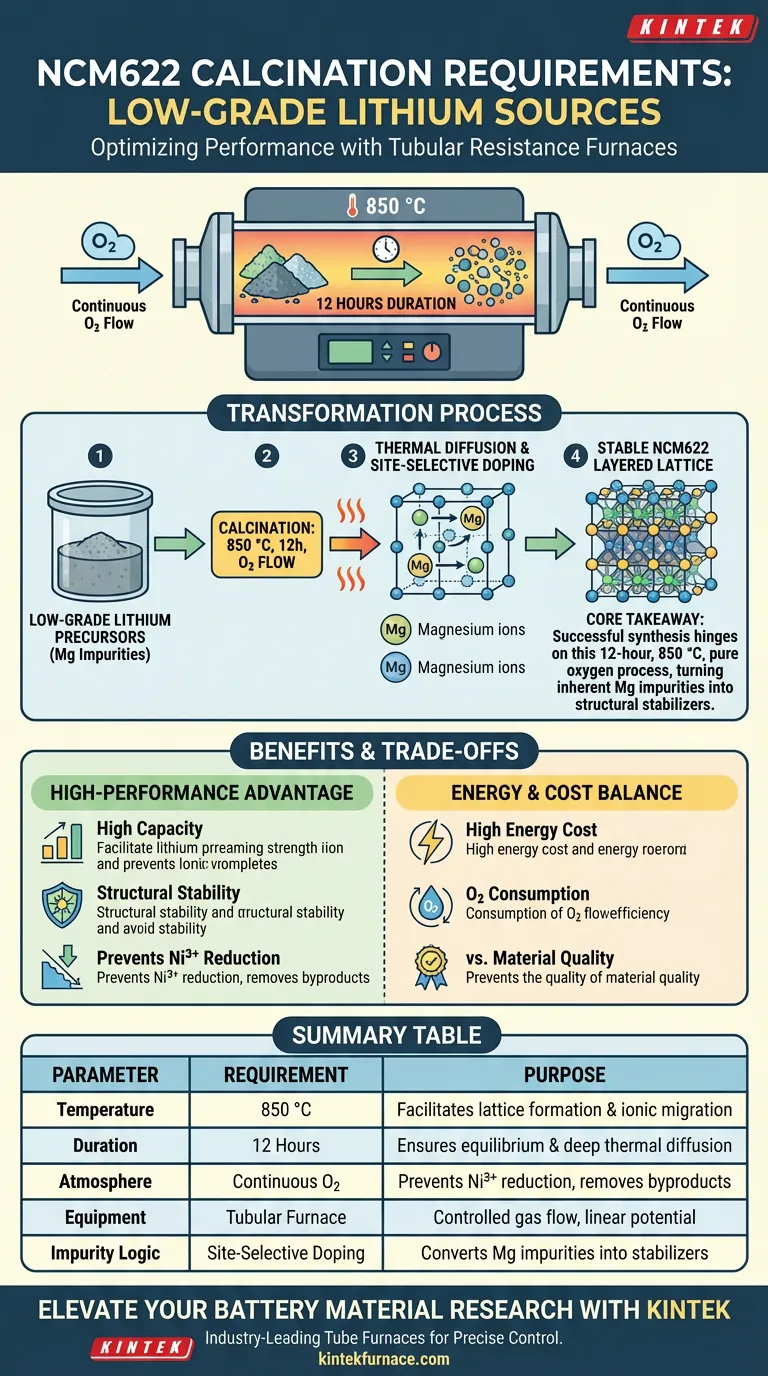
Calcination of NCM622 cathode materials requires precise thermal and atmospheric control to transform low-grade lithium precursors into high-performance battery components. Specifically, you must operate a tubular resistance furnace at 850 °C for a duration of 12 hours under a continuous oxygen (O2) flow. This environment facilitates the complete reaction between lithium salts and transition metal precursors, ensuring the formation of a highly ordered layered lattice structure.
Core Takeaway: Successful synthesis of NCM622 from low-grade sources hinges on a 12-hour, 850 °C calcination cycle in a pure oxygen atmosphere. This specific process uses high-temperature thermal diffusion to convert inherent magnesium impurities into beneficial site-selective dopants, stabilizing the material’s crystal structure.

Thermal Parameters for NCM622 Synthesis
Precise Temperature Control at 850 °C
The furnace must be maintained at a steady 850 °C to provide the kinetic energy necessary for lattice formation. This temperature is the threshold where transition metal ions and lithium ions migrate into their designated positions within the layered oxide framework.
Failure to reach this temperature can result in incomplete lithiation, while exceeding it may lead to excessive grain growth or primary particle sintering.
The Critical 12-Hour Duration
A residence time of 12 hours is required to ensure the reaction reaches equilibrium across the entire batch. This extended period allows for the "soaking" of the material, ensuring that the core of the precursor particles reacts as thoroughly as the surface.
This duration is particularly important when using low-grade sources, as it provides ample time for the redistribution of various ionic species throughout the material.
The Role of the Oxygen Atmosphere
Maintaining Continuous O2 Flow
A continuous flow of oxygen within the tubular resistance furnace is non-negotiable for NCM622 production. The oxygen atmosphere prevents the reduction of nickel ions (Ni3+ to Ni2+), which is essential for maintaining high discharge capacity and structural stability.
The flow also helps sweep away any gaseous byproducts generated during the decomposition of lithium salts, preventing localized pressure build-ups that could disrupt the crystalline structure.
Optimizing the Internal Environment
The tubular design of the furnace is ideal for this process because it allows for a controlled, linear path for gas flow. This ensures that every part of the material is exposed to a consistent chemical potential of oxygen throughout the 12-hour cycle.
Addressing Low-Grade Lithium Challenges
Managing Magnesium Impurities
Low-grade lithium sources often contain magnesium, which can negatively impact performance if not managed correctly. The high-temperature environment of 850 °C leverages these impurities by encouraging thermal diffusion.
Instead of remaining as a detrimental impurity, the magnesium ions are guided into specific lattice positions through this diffusion process.
Site-Selective Doping
This process effectively turns a "low-grade" challenge into a "high-performance" advantage through site-selective doping. By precisely controlling the calcination requirements, the magnesium ions occupy positions that reinforce the layered structure.
This unique doping mechanism is a direct result of the specific thermal profile and is critical for stabilizing the NCM622 framework against degradation during cycling.
Understanding the Trade-offs
Balancing Temperature and Energy Costs
While 850 °C is optimal for structural integrity, maintaining this temperature for 12 hours represents a significant energy expenditure. Lowering the temperature or time might reduce costs but risks "cation mixing," where nickel ions occupy lithium sites, severely degrading battery performance.
Oxygen Consumption vs. Material Purity
The requirement for continuous O2 flow increases operational complexity and cost compared to air calcination. However, using ambient air is generally insufficient for NCM622, as the lower oxygen partial pressure leads to an increase in oxygen vacancies and structural defects.
How to Apply This to Your Project
When configuring your tubular resistance furnace for NCM622 production, your approach should vary based on your specific quality and throughput targets.
- If your primary focus is Maximum Structural Stability: Adhere strictly to the 12-hour duration at 850 °C to ensure magnesium ions are fully integrated via site-selective doping.
- If your primary focus is Utilizing High-Impurity Sources: Ensure the O2 flow rate is high enough to aggressively remove byproducts and maintain a highly oxidizing environment throughout the calcination.
- If your primary focus is Throughput Optimization: Do not reduce the temperature below 850 °C; instead, focus on optimizing the ramp-up and cool-down phases of the furnace to shave off total cycle time without compromising the 12-hour "soak."
By mastering these specific calcination requirements, you transform low-grade lithium precursors into a robust, high-capacity NCM622 cathode material.
Summary Table:
| Parameter | Requirement | Purpose |
|---|---|---|
| Temperature | 850 °C | Facilitates lattice formation and ionic migration |
| Duration | 12 Hours | Ensures equilibrium and deep thermal diffusion |
| Atmosphere | Continuous Oxygen (O2) | Prevents Ni3+ reduction and removes gaseous byproducts |
| Equipment | Tubular Furnace | Provides controlled gas flow and linear chemical potential |
| Impurity Logic | Site-Selective Doping | Converts magnesium impurities into structural stabilizers |
Elevate Your Battery Material Research with KINTEK
Achieving the precise 850°C thermal profile and oxygen control required for NCM622 synthesis demands high-performance equipment. KINTEK provides industry-leading Tube, Muffle, and Vacuum furnaces designed specifically for sensitive chemical reactions and material science.
Why choose KINTEK?
- Expert R&D & Manufacturing: Our systems offer the thermal stability and atmospheric precision necessary to turn low-grade precursors into high-performance cathodes.
- Customizable Solutions: Whether you need specific gas flow configurations or rotary systems for bulk processing, we tailor our hardware to your unique lab needs.
Contact KINTEK today to discuss your furnace requirements and ensure your next batch reaches peak structural stability.
Visual Guide
References
- Gogwon Choe, Yong‐Tae Kim. Re-evaluation of battery-grade lithium purity toward sustainable batteries. DOI: 10.1038/s41467-024-44812-3
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role does the high-temperature vacuum tube furnace play in SiC/SiC pyrolysis? Essential Chemical Transformation
- What is flash vacuum pyrolysis and how is a tube furnace utilized in this process? Unlock High-Temp Chemical Reactions
- What are the objectives of using a tube furnace for dual-layer nanocomposite heat treatment? Maximize Coating Stability
- Why must the final sintering of NiTiNb alloys be conducted in a high-vacuum tube furnace? Ensure Pure Shape Memory Performance
- How does gas flow control in a tube furnace influence the quality of NMC811? Master Stable Calcination Environments
- How can the performance of a vertical tube furnace be optimized? Boost Efficiency and Precision in Heat Treatment
- What types of tube materials are available for tube furnaces and what are their temperature limits? Choose the Right Material for Your Lab
- What makes fluidized bed vertical tube furnaces environmentally friendly? Discover Efficient Green Tech Solutions



















