At its core, Flash Vacuum Pyrolysis (FVP) is a powerful chemical technique used to break down substances by exposing them to extremely high temperatures for a very short duration in a high-vacuum environment. A tube furnace is the essential piece of equipment that creates this precise, high-temperature reaction zone, typically housing a quartz tube through which the precursor material passes and decomposes.
The central purpose of FVP is not just to break molecules apart, but to do so under conditions so extreme and brief that it allows chemists to generate and isolate highly reactive, unstable molecules that cannot be formed or studied using conventional methods.

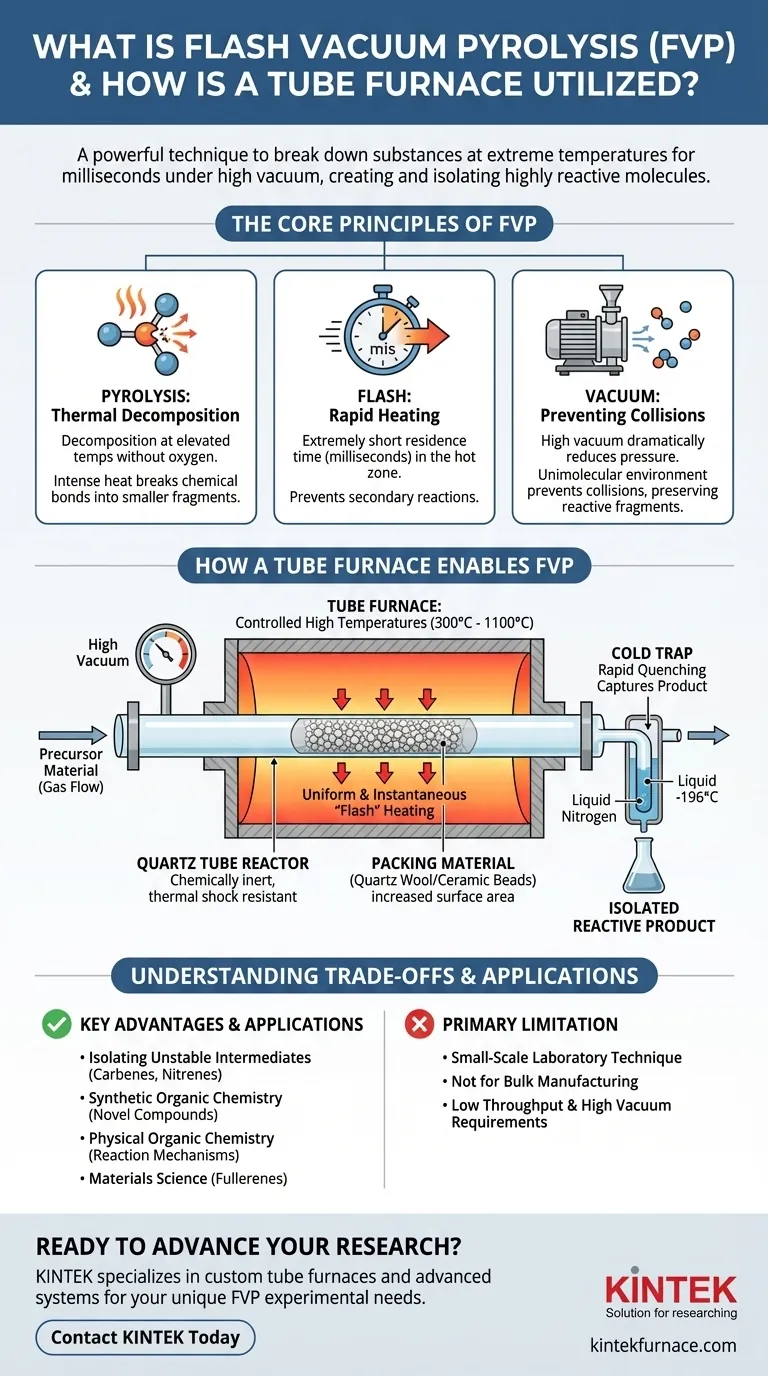
The Core Principles of Flash Vacuum Pyrolysis
To understand the process, it's best to break down its name into three key components: Pyrolysis, Flash, and Vacuum.
Pyrolysis: Thermal Decomposition
Pyrolysis is the thermal decomposition of materials at elevated temperatures in the absence of oxygen.
Instead of burning (combustion), the intense heat provides enough energy to break the chemical bonds within a molecule, causing it to fragment into smaller, often simpler, molecules.
The "Flash" Component: Rapid Heating
The term "flash" refers to the extremely short residence time of the chemical precursor in the hot zone of the furnace. This is typically on the order of milliseconds.
This rapid heating and immediate exit from the hot zone are critical. It ensures that the initial products of decomposition are formed but do not have time to undergo further, secondary reactions, which would lead to a complex and undesirable mixture of byproducts.
The "Vacuum" Component: Preventing Collisions
The entire process is conducted under a high vacuum. This dramatically reduces the pressure inside the apparatus, minimizing the number of gas molecules present.
By removing other molecules, the vacuum prevents the newly formed, highly reactive product fragments from colliding with anything else. This unimolecular environment is essential for preserving their structure long enough to be collected and studied.
How a Tube Furnace Enables FVP
The tube furnace is the heart of the FVP apparatus, providing the precisely controlled environment needed for the reaction to occur.
Providing Controlled, High Temperatures
The primary function of the tube furnace is to generate and maintain the high temperatures required for pyrolysis, often ranging from 300°C to over 1100°C.
This temperature must be stable and uniform across the reaction zone to ensure consistent and predictable decomposition of the starting material.
The Quartz Tube Reactor
The reaction itself takes place inside a tube, typically made of fused quartz, that passes through the center of the furnace.
Quartz is the material of choice because it is chemically inert at high temperatures and is highly resistant to thermal shock, preventing it from cracking under the extreme temperature gradients.
Packing Material: Maximizing Surface Area
To ensure efficient and rapid heat transfer, the quartz tube is often packed with an inert material like quartz wool or ceramic beads.
As the gaseous precursor flows through the packed tube, it is forced into contact with a large, hot surface area. This guarantees the "flash" heating is uniform and instantaneous, which is crucial for the success of the technique.
The Cold Trap: Capturing the Product
Immediately after exiting the furnace, the gas stream is directed onto an extremely cold surface, such as a "cold finger" chilled with liquid nitrogen (-196°C).
This rapid quenching causes the reactive product to freeze solid, trapping it in an isolated state before it has a chance to decompose or react. The product can then be analyzed or used in subsequent reactions.
Understanding the Trade-offs and Applications
FVP is a specialized technique with distinct advantages and clear limitations.
Key Advantage: Isolating Unstable Intermediates
The primary power of FVP lies in its ability to generate and study reaction intermediates and other unstable species. Molecules like carbenes, nitrenes, and strained cyclic compounds can be produced and characterized in a way that is impossible in solution.
Common Applications
FVP is widely used in synthetic organic chemistry to create novel compounds. It is also a key tool in physical organic chemistry for studying reaction mechanisms and in materials science for synthesizing molecules like fullerenes.
The Primary Limitation: Scale
FVP is fundamentally a small-scale laboratory technique. The requirements for high vacuum and the low throughput mean it is not suitable for producing large quantities of material. Its value is in discovery and analysis, not bulk manufacturing.
Making the Right Choice for Your Goal
When deciding if FVP is appropriate, consider your ultimate research objective.
- If your primary focus is synthesizing highly reactive molecules: FVP is one of the premier techniques for generating and trapping species that are too unstable to exist under normal laboratory conditions.
- If your primary focus is studying unimolecular reaction pathways: The low-pressure environment of FVP is ideal for observing how a single molecule behaves when subjected to thermal energy, free from intermolecular effects.
- If your primary focus is large-scale production: You should investigate alternative synthetic routes, as FVP is inherently a low-yield, high-energy process designed for analytical or small-scale preparative work.
Ultimately, mastering Flash Vacuum Pyrolysis gives you a powerful tool to explore the frontiers of chemical reactivity by creating conditions where the unstable can be made stable.
Summary Table:
| Aspect | Description |
|---|---|
| Process | Flash Vacuum Pyrolysis (FVP) breaks down substances at high temperatures briefly in a vacuum to isolate reactive intermediates. |
| Tube Furnace Role | Provides controlled high temperatures (300°C to 1100°C) in a quartz tube for uniform decomposition and rapid heating. |
| Key Components | Quartz tube, inert packing material, cold trap for product collection. |
| Applications | Synthetic organic chemistry, physical organic chemistry, materials science (e.g., fullerenes). |
| Limitations | Small-scale technique, not suitable for bulk production due to high vacuum and low throughput. |
Ready to advance your chemical research with precision high-temperature solutions? KINTEK specializes in custom tube furnaces and other advanced systems like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Leveraging our strong R&D and in-house manufacturing, we deliver tailored solutions to meet your unique experimental needs in FVP and beyond. Contact us today to discuss how we can enhance your lab's capabilities and drive innovation!
Visual Guide
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- How does a vertical tube furnace facilitate the simulation of the industrial sintering process for iron ores?
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- What function does a tube furnace serve in the PVT growth of J-aggregate molecular crystals? Mastery of Thermal Control
- How do roller kilns and tube furnaces differ in their use of Alumina ceramic tubes? Compare Transport vs. Containment
- What are the material requirements for furnace tubes? Optimize Performance and Safety in High-Temperature Labs



















