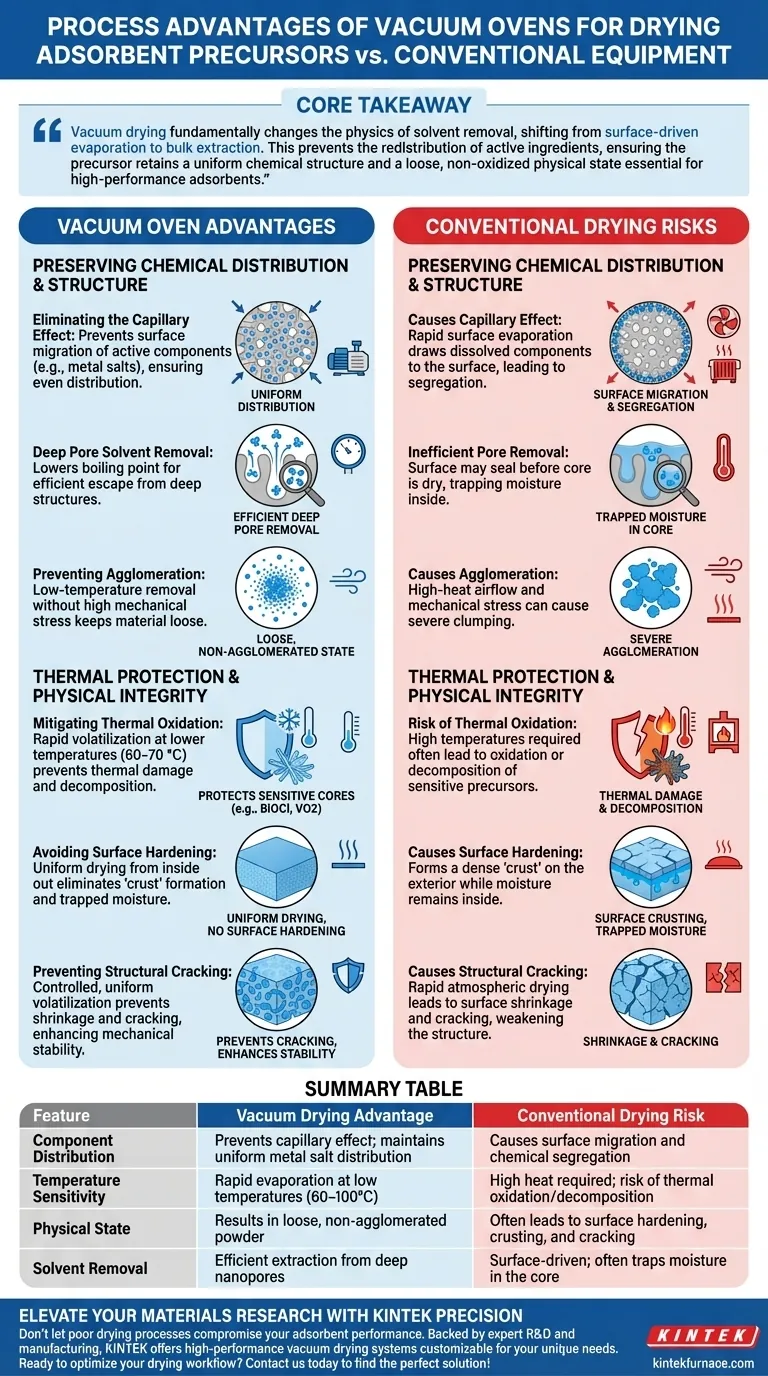
The primary advantage of using a vacuum oven is the preservation of internal component distribution. By creating a low-pressure environment, a vacuum oven allows solvents to evaporate rapidly from deep pores at lower temperatures (e.g., 60–100 °C). This process eliminates the "capillary effect" common in conventional drying, ensuring that active components do not migrate to the surface and preventing the segregation of metal salts within the adsorbent precursor.
Core Takeaway Vacuum drying fundamentally changes the physics of solvent removal, shifting from surface-driven evaporation to bulk extraction. This prevents the redistribution of active ingredients, ensuring the precursor retains a uniform chemical structure and a loose, non-oxidized physical state essential for high-performance adsorbents.

Preserving Chemical Distribution and Structure
The most critical process advantage, as highlighted by the primary reference, relates to how active components behave during the drying phase.
Eliminating the Capillary Effect
In conventional atmospheric drying, rapid surface evaporation draws liquid from the interior to the exterior. This flow, known as the capillary effect, carries dissolved active components (such as metal salts) along with it.
This migration causes these components to concentrate on the outer surface of the material. Vacuum drying disrupts this mechanism, ensuring components remain evenly distributed throughout the precursor.
Deep Pore Solvent Removal
Adsorbent precursors often rely on porous carriers with deep internal structures. Conventional heat may seal the surface before the core is dry.
A vacuum environment lowers the boiling point of the solvent, allowing it to escape from these deep pores efficiently. This ensures the "distributional stability" of the active components inside the pore structure, rather than just on the surface.
Preventing Agglomeration
Drying in a vacuum oven helps maintain the physical state of the precursor powder.
By removing solvents at lower temperatures without the mechanical stress of high-heat airflow, the material resists severe agglomeration. This results in a "loose" physical state, which is ideal for subsequent processing steps like solid-state sintering.
Thermal Protection and Physical Integrity
Beyond chemical distribution, the vacuum process offers distinct advantages regarding the thermal history and physical durability of the material.
Mitigating Thermal Oxidation
Many precursors, such as BiOCl or VO2 cores, are sensitive to high temperatures and oxygen exposure.
By reducing the pressure, vacuum ovens allow solvents (like water, ethanol, or NMP) to volatilize rapidly at significantly lower temperatures (e.g., 60–70 °C). This prevents the thermal oxidation or decomposition that often occurs when attempting to drive off solvents using heat alone.
Avoiding Surface Hardening
Conventional drying often leads to a "crust" forming on the exterior of the sample while moisture remains trapped inside.
The vacuum environment prevents this surface hardening. By ensuring uniform drying from the inside out, it eliminates the risk of internal moisture becoming trapped, which can be detrimental during later calcination stages.
Preventing Structural Cracking
For applications involving slurries or binders, rapid atmospheric drying can cause the surface layer to shrink and crack.
Vacuum drying facilitates a controlled, uniform volatilization. This prevents cracking and ensures a uniform distribution of binders, thereby enhancing the mechanical stability of the final electrode or adsorbent structure.
Understanding the Trade-offs
While vacuum drying is superior for quality, it is important to understand the specific dynamics it introduces compared to conventional methods.
The Risk of Conventional "Crusting"
The primary pitfall of not using a vacuum oven is the creation of a density gradient.
Conventional ovens rely on heat transfer that dries the outside first. This frequently results in a dense, component-rich shell and a hollow or chemically depleted core. This structural inconsistency is often irreversible.
Vacuum Process Control
While vacuum drying prevents oxidation, it requires precise pressure management.
If the pressure drops too suddenly without temperature control, solvents can flash-boil, potentially disrupting delicate nanostructures. However, when managed correctly, it is the only method that guarantees the thorough removal of trace solvents from nanopores without thermal damage.
Making the Right Choice for Your Goal
To determine if a vacuum oven is strictly necessary for your specific precursor, consider your primary performance metrics.
- If your primary focus is Chemical Uniformity: Use a vacuum oven to prevent the capillary effect and ensure metal salts remain evenly distributed within the carrier pores.
- If your primary focus is Material Purity: Rely on vacuum drying to lower the evaporation temperature, preventing oxidation or decomposition of heat-sensitive cores (like VO2 or BiOCl).
- If your primary focus is Physical Handling: Choose vacuum drying to prevent agglomeration and surface hardening, ensuring the powder remains loose and ready for sintering.
Summary: The vacuum oven is not merely a drying tool; it is a structural preservation device that decouples solvent removal from thermal stress to maintain the intrinsic quality of the precursor.
Summary Table:
| Feature | Vacuum Drying Advantage | Conventional Drying Risk |
|---|---|---|
| Component Distribution | Prevents capillary effect; maintains uniform metal salt distribution | Causes surface migration and chemical segregation |
| Temperature Sensitivity | Rapid evaporation at low temperatures (60–100°C) | High heat required; risk of thermal oxidation/decomposition |
| Physical State | Results in loose, non-agglomerated powder | Often leads to surface hardening, crusting, and cracking |
| Solvent Removal | Efficient extraction from deep nanopores | Surface-driven; often traps moisture in the core |
Elevate Your Materials Research with KINTEK Precision
Don’t let poor drying processes compromise your adsorbent performance. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum drying systems, Muffle, Tube, Rotary, and CVD systems, all customizable for your unique laboratory needs. Whether you are preserving sensitive precursors or ensuring chemical uniformity, our equipment provides the control you need to succeed.
Ready to optimize your drying workflow? Contact us today to find the perfect solution!
Visual Guide
References
- Zhiyuan Liu, Guoqiang Huang. Acid-modified Cu–Ce/HZSM-5 adsorbent removes trace phosphorus impurities from recycled hydrogen during polysilicon production. DOI: 10.1039/d5ra01322d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- What is the purpose of using a continuous annealing furnace? Optimize Silicon Steel Normalization & Performance
- What are the advantages of using a precision vacuum drying oven? Master Ceramic Powder Treatment with KINTEK
- What role does a pyrolysis furnace play in preparing graphene nanosheets? Master High-Value Plastic Transformation
- What role does the addition of NaCl as a diluent play in the SHS of Titanium Diboride? Master Nano-Powder Synthesis
- How does the 1600°C range influence biomass microstructure? Transform Carbon into High-Performance Graphite
- Why is it necessary to dry Industrial EAF slag before hydrogen reduction? Crucial Safety and Accuracy Prep
- Why is calcination at 700 °C necessary for extracted diatomaceous biosilica? Achieve Peak Material Stability
- What is the necessity of the subsequent pyrolysis step in ZnS-CFC preparation? Unlocking High-Performance Carbonization



















