High-purity Argon (Ar) acts as the fundamental regulator of the Chemical Vapor Deposition (CVD) environment, serving simultaneously as a transport vehicle and a protective shield. In the synthesis of Mn2P4O12, this inert gas is essential for moving reactants between thermal zones and maintaining the chemical integrity of the system against atmospheric contamination.
In CVD phosphorization, Argon is the primary control lever for reaction kinetics and purity. It quantitatively transports phosphorus vapor to the reaction site while creating an inert environment to prevent oxidation, ensuring the formation of pure-phase Mn2P4O12.

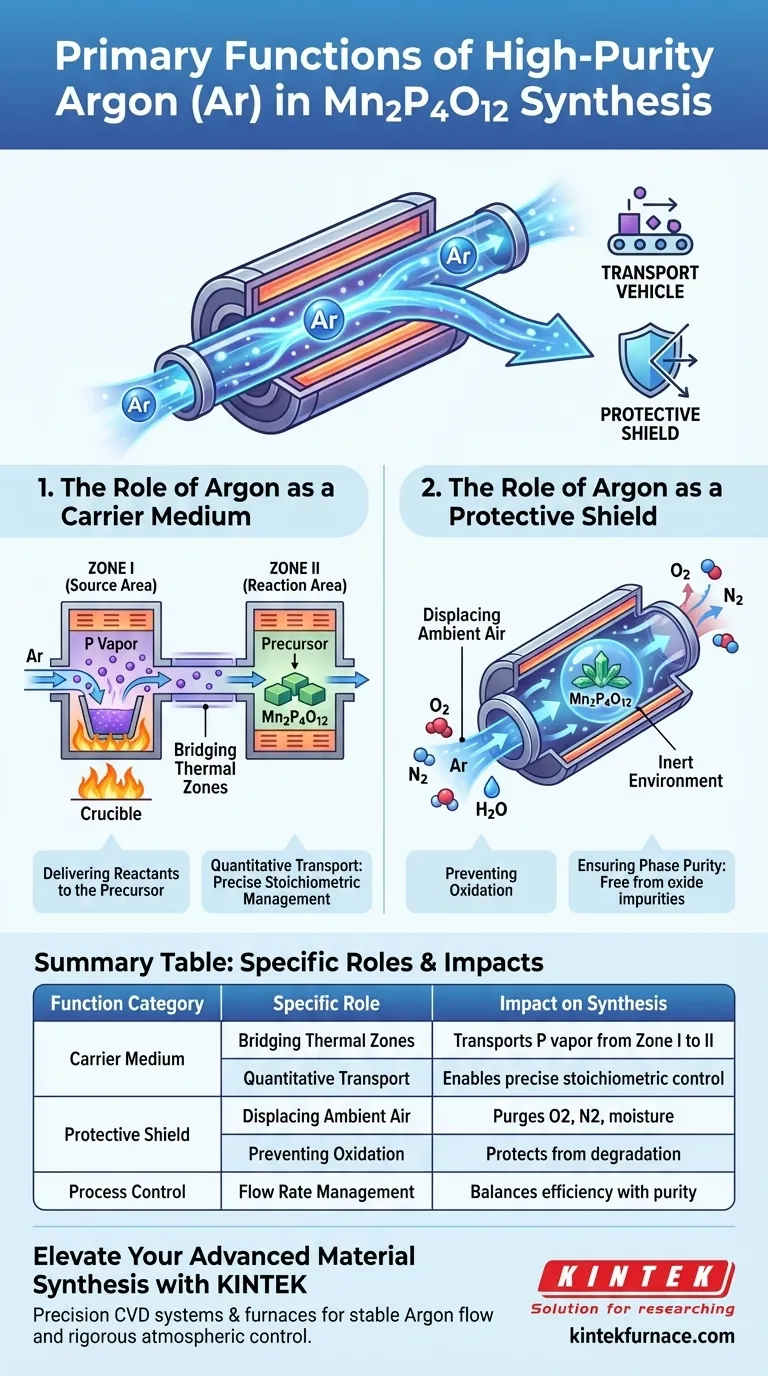
The Role of Argon as a Carrier Medium
To synthesize Mn2P4O12 effectively, reactants must be moved precisely within the furnace. Argon provides the motive force for this transfer.
Bridging Thermal Zones
The synthesis process utilizes a multi-zone setup. Argon acts as a carrier gas, picking up phosphorus vapor generated in the source area (Zone I).
Delivering Reactants to the Precursor
Once loaded with phosphorus vapor, the Argon stream flows into the reaction area (Zone II). Here, it delivers the phosphorus to react with the precursors.
Quantitative Transport
The flow of Argon does not just move material; it ensures quantitative transport. This means the amount of phosphorus reaching the reaction zone can be controlled by the gas flow, allowing for precise stoichiometric management.
The Role of Argon as a Protective Shield
High-temperature synthesis renders materials highly susceptible to contamination. Argon provides the necessary isolation from the outside environment.
Displacing Ambient Air
Before and during the reaction, the Argon flow physically displaces air within the furnace tube. This purging process removes nitrogen, oxygen, and moisture that naturally exist in the atmosphere.
Preventing Oxidation
The primary threat to Mn2P4O12 synthesis is unintended oxidation. Without an inert atmosphere, the phosphorus source would degrade before reaching the precursor.
Ensuring Phase Purity
By maintaining a strictly inert environment, Argon protects both the reactants and the resulting products. This isolation is the critical factor in obtaining a pure-phase Mn2P4O12, free from oxide impurities or secondary phases.
Operational Considerations and Trade-offs
While Argon flow is necessary, it must be carefully managed to avoid process inefficiencies.
Flow Rate Precision
The term "quantitative transport" implies a direct link between flow rate and material delivery. If the flow is inconsistent, the stoichiometry of the reaction in Zone II will fluctuate, potentially leading to incomplete reactions.
Gas Purity Dependencies
The protection offered is only as good as the gas source. If the "high-purity" Argon contains trace moisture or oxygen, the protective atmosphere is compromised, regardless of the flow rate.
Optimizing Your Synthesis Strategy
To achieve the best results in Mn2P4O12 synthesis, align your Argon management with your specific process goals.
- If your primary focus is Reaction Efficiency: Calibrate the Argon flow rate to ensure optimal transport speed from Zone I to Zone II, preventing reactant starvation at the precursor site.
- If your primary focus is Material Purity: Prioritize the integrity of the system seal and the grade of Argon used to ensure the complete displacement of air and total prevention of oxidation.
Mastering the Argon flow is the key to balancing efficient reactant delivery with the strict atmospheric control required for high-quality Mn2P4O12.
Summary Table:
| Function Category | Specific Role | Impact on Mn2P4O12 Synthesis |
|---|---|---|
| Carrier Medium | Bridging Thermal Zones | Transports phosphorus vapor from Zone I to Zone II |
| Carrier Medium | Quantitative Transport | Enables precise stoichiometric control of reactants |
| Protective Shield | Displacing Ambient Air | Purges O2, N2, and moisture from the furnace tube |
| Protective Shield | Preventing Oxidation | Protects precursors and products from degradation |
| Process Control | Flow Rate Management | Balances reaction efficiency with material phase purity |
Elevate Your Advanced Material Synthesis with KINTEK
Precision in Mn2P4O12 synthesis requires more than just gas—it demands a high-performance thermal environment. KINTEK provides industry-leading CVD systems, Muffle, Tube, and Vacuum furnaces designed to deliver the stable Argon flow and rigorous atmospheric control your research requires.
Backed by expert R&D and manufacturing, our systems are fully customizable to meet your unique stoichiometric and purity needs. Ensure the integrity of your next project with KINTEK’s reliable high-temperature solutions.
Contact Our Experts Today to Optimize Your Lab Setup
Visual Guide
References
- Kassa Belay Ibrahim, Alberto Vomiero. Electrochemically Modified Mn₂P₄O₁₂ as an Emerging Catalyst for Oxygen Evolution Reaction. DOI: 10.1002/admi.202500216
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Magnesium Extraction and Purification Condensing Tube Furnace
- Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
People Also Ask
- How does the CVD process alter substrate properties? Enhance Durability, Conductivity, and More
- What is the purpose of methane in CVD graphene growth? Master the Key Carbon Source for High-Quality Graphene
- Why is the tube design important in CVD furnaces? Ensure Uniform Deposition for High-Quality Films
- What are the typical thickness ranges for coatings produced by CVD compared to traditional deposition methods? Discover Precision vs. Bulk
- What advantages do CVD coatings provide for sub-micron filters? Enhance Purity and Durability in Filtration
- How is CVD applied in the production of solar cells? Enhance Efficiency with Precision Film Deposition
- What limitations does CVD have in coating large surfaces? Overcome Scale Challenges with Custom Solutions
- What are the advantages of chemical vapour deposition? Achieve Superior, Uniform Coatings on Complex 3D Surfaces



















