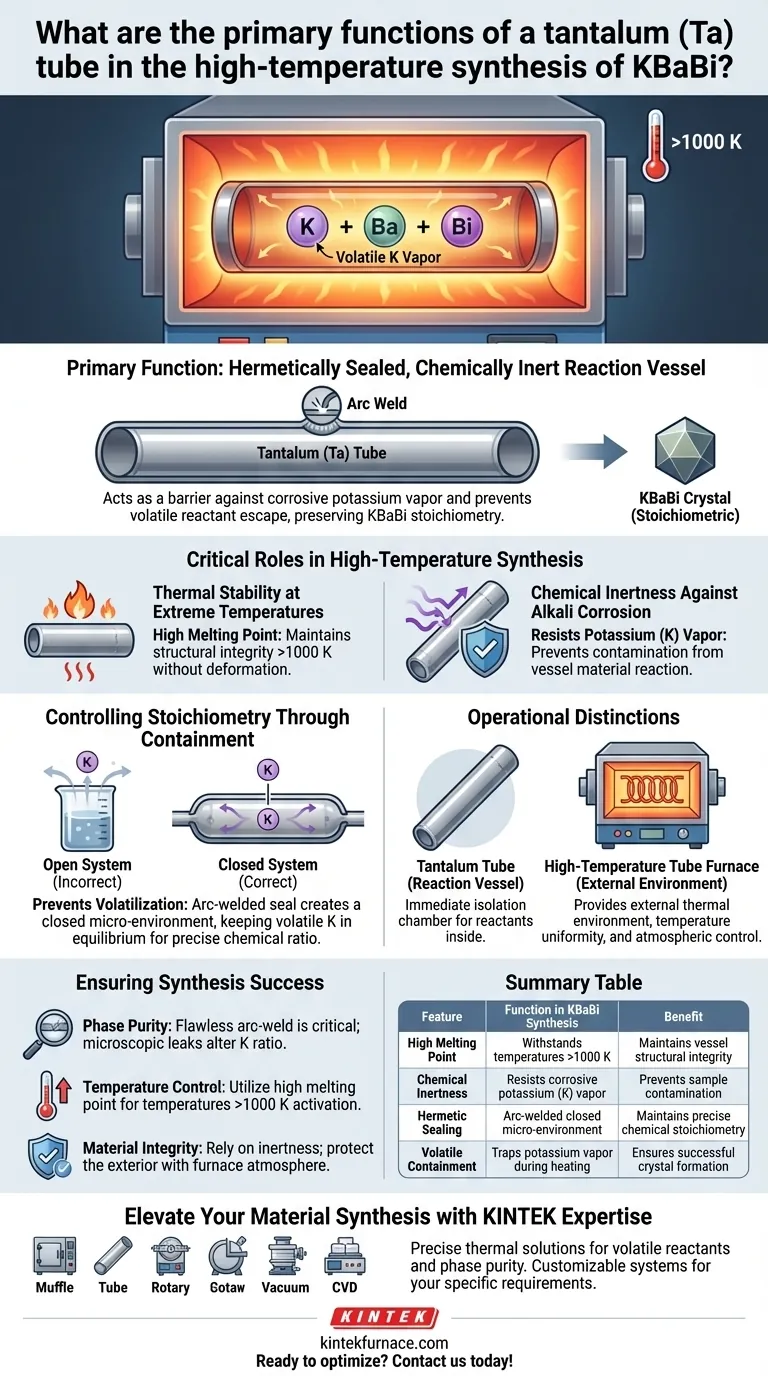
The primary function of a tantalum (Ta) tube is to serve as a hermetically sealed, chemically inert reaction vessel that withstands temperatures exceeding 1000 K. In the specific synthesis of KBaBi, it prevents the corrosive attack of potassium vapor and stops the volatile alkali metal from escaping, thereby preserving the exact chemical stoichiometry required for the reaction.
The success of KBaBi synthesis relies on the tantalum tube's ability to act as a barrier. It creates a closed micro-environment that contains volatile reactants while resisting the extreme corrosive nature of alkali metal vapors at high heat.

The Critical Role of Tantalum in High-Temperature Synthesis
Thermal Stability at Extreme Temperatures
The synthesis of KBaBi requires significant thermal activation energy to drive the reaction.
Tantalum is selected specifically for its high melting point, which allows the vessel to maintain structural integrity at synthesis temperatures well above 1000 K.
This thermal resilience ensures the container does not deform or fail during the heating process provided by the external furnace.
Chemical Inertness Against Alkali Corrosion
At high temperatures, potassium (K) vapor becomes highly corrosive to most standard container materials.
Tantalum possesses excellent chemical inertness specifically against these high-temperature alkali metal vapors.
By resisting corrosion, the tube prevents the contamination of the KBaBi sample that would occur if the vessel material reacted with the potassium.
Controlling Stoichiometry Through Containment
Preventing Volatilization
Potassium is a volatile element that readily turns to vapor at synthesis temperatures.
If the reaction were performed in an open system, the potassium would evaporate, destroying the chemical stoichiometry (the precise ratio of elements) necessary to form KBaBi.
Creating a Closed Micro-Environment
To counter volatilization, the tantalum tube is sealed using arc welding.
This welding process creates a hermetically sealed, closed micro-environment.
Within this sealed space, the potassium vapor is trapped in equilibrium with the solids, ensuring that the final product retains the correct chemical composition.
Operational Distinctions and Requirements
The Difference Between Vessel and Furnace
It is critical to distinguish between the tantalum tube and the high-temperature tube furnace.
The furnace provides the external thermal environment, temperature uniformity, and atmospheric control (such as vacuum or inert gas) to protect the exterior of the apparatus.
The tantalum tube, placed inside the furnace, acts as the immediate isolation chamber for the reactants themselves.
The Integrity of the Seal
The efficacy of this method relies entirely on the quality of the arc weld.
Because the system is closed, any breach in the weld will result in the immediate loss of potassium vapor.
This loss leads to phase impurities and an incomplete crystal structure, negating the benefits of using tantalum.
Ensuring Synthesis Success
To maximize the quality of your KBaBi synthesis, consider the following based on your specific objectives:
- If your primary focus is Phase Purity: Ensure the tantalum tube is flawlessly arc-welded to create a perfect seal, as even microscopic leaks will alter the potassium ratio.
- If your primary focus is Temperature Control: Utilize the high melting point of tantalum to push synthesis temperatures above 1000 K without fear of vessel failure, ensuring sufficient activation energy.
- If your primary focus is Material Integrity: Rely on tantalum's inertness to prevent corrosion-based contamination, but ensure the external furnace atmosphere protects the outside of the tantalum tube itself.
By leveraging the dual properties of thermal resistance and chemical inertness, the tantalum tube effectively turns a volatile, corrosive mixture into a stable, stoichiometric compound.
Summary Table:
| Feature | Function in KBaBi Synthesis | Benefit |
|---|---|---|
| High Melting Point | Withstands temperatures >1000 K | Maintains vessel structural integrity |
| Chemical Inertness | Resists corrosive potassium (K) vapor | Prevents sample contamination |
| Hermetic Sealing | Arc-welded closed micro-environment | Maintains precise chemical stoichiometry |
| Volatile Containment | Traps potassium vapor during heating | Ensures successful crystal formation |
Elevate Your Material Synthesis with KINTEK Expertise
Precise high-temperature reactions like KBaBi synthesis require the perfect synergy between reaction vessels and furnace performance. KINTEK provides the advanced thermal solutions necessary to protect your volatile reactants and ensure phase purity.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to provide the precise temperature uniformity and atmospheric control your tantalum-sealed experiments demand.
Ready to optimize your lab's high-temp capabilities? Contact us today to discuss your unique synthesis requirements with our technical team!
Visual Guide
References
- Investigation of a Ternary Zintl Phase KBaBi: Synthesis, Crystal Structure, and Preliminary Transport Properties. DOI: 10.1002/zaac.202500064
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the properties and uses of pure platinum as a heating element? Ideal for High-Temp Precision and Purity
- Why is a high-precision thermocouple probe used during the temperature calibration of fiber optic sensors? Guide
- What precautions should be taken when replacing SiC resistors? Ensure Safe, Long-Lasting Performance
- Why must air cooling be integrated into acoustic emission sensors for high-temp experiments? Protect Your Data Integrity
- How do silicon carbide heating elements reduce operating costs? Achieve Long-Term Savings and Efficiency
- What are the unique properties and applications of platinum in heating elements? Discover Its High-Temp Reliability
- What is a heating element and what is its primary function? Discover Efficient Heat Generation for Your Applications
- How do in-situ heaters and precision current sources cooperate to stabilize the SkBL in NdMn2Ge2?



















