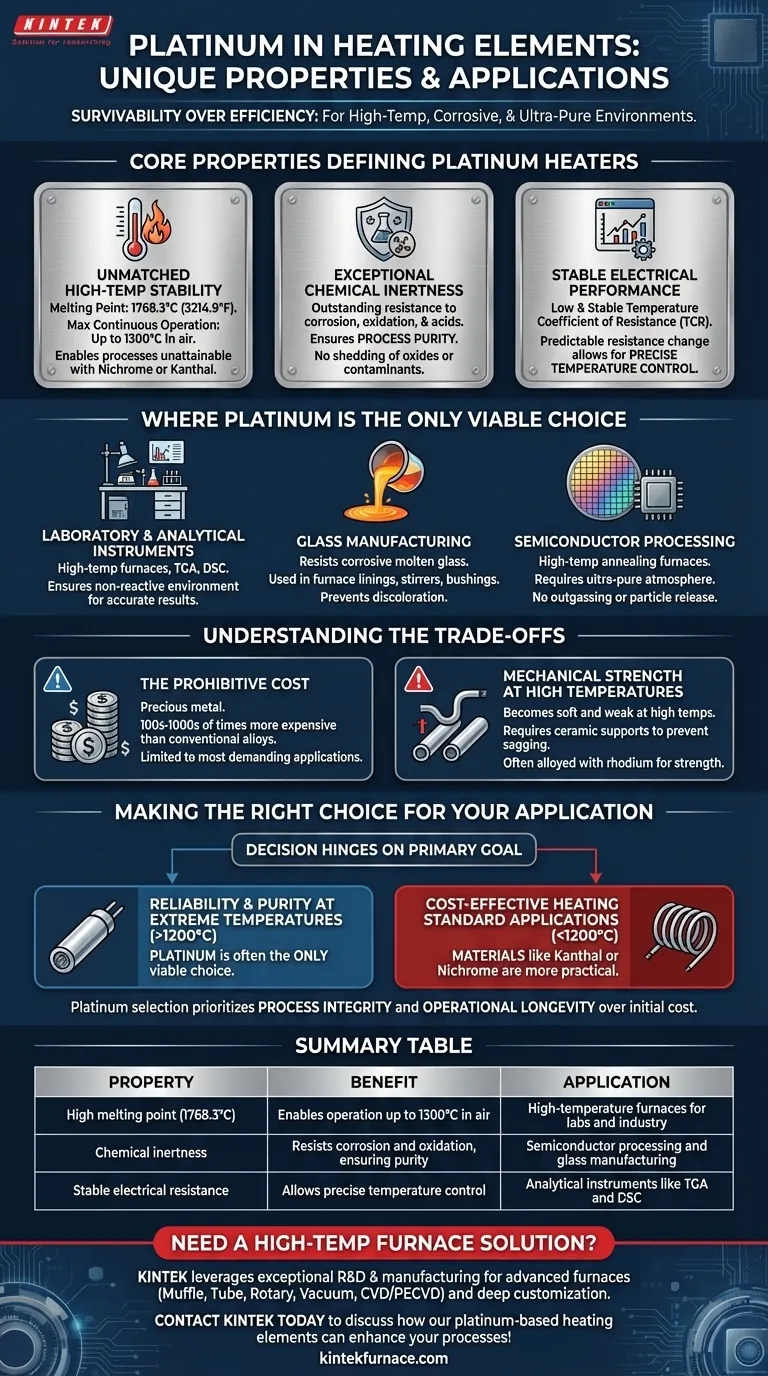
At its core, platinum is used in heating elements for its unique combination of an extremely high melting point, chemical inertness, and stable electrical properties. This allows it to operate reliably in high-temperature, corrosive environments where less robust materials would quickly degrade or contaminate the process.
Platinum is not chosen for its efficiency as a heater, but for its survivability. The decision to use it is driven by applications where process purity and operational reliability in extreme conditions are more critical than the initial material cost.

The Core Properties Defining Platinum Heaters
To understand why platinum is reserved for such specific duties, we must examine its three defining characteristics. Each one solves a problem that more common heating element materials cannot.
Unmatched High-Temperature Stability
Platinum possesses a very high melting point of 1768.3°C (3214.9°F). This fundamental property allows for a maximum continuous operating temperature of up to 1300°C in air.
This capability enables thermal processes that are simply unattainable with common alloys like Nichrome or Kanthal, which typically fail or degrade rapidly above 1200°C.
Exceptional Chemical Inertness
Platinum exhibits outstanding resistance to corrosion and oxidation, even at extreme temperatures. It does not readily react with air, water, or most acids.
This chemical stability is critical in applications where purity is paramount. A platinum heater will not shed oxides or other contaminants into the environment, which is essential for manufacturing sensitive materials like semiconductors, optical glass, or high-purity crystals.
Stable and Predictable Electrical Performance
Platinum has a low and stable temperature coefficient of resistance (TCR). This means its electrical resistance changes in a highly predictable and linear fashion as it heats up.
This predictability is invaluable for sophisticated equipment that requires precise temperature control. By accurately monitoring the element's resistance, control systems can maintain a set temperature with exceptional accuracy.
Where is Platinum the Only Viable Choice?
The combination of these properties makes platinum indispensable in a few high-stakes industries where failure or contamination carries a significant cost.
Laboratory and Analytical Instruments
High-temperature laboratory furnaces, thermogravimetric analyzers (TGA), and differential scanning calorimeters (DSC) rely on platinum heaters. The chemical inertness ensures that experimental results are not skewed by reactions between the heater and the sample being analyzed.
Glass Manufacturing
Molten glass is extremely corrosive to most metals. Platinum is one of the few materials that can contain and heat molten glass without being dissolved or introducing impurities that would discolor the final product. It is used for furnace linings, stirrers, and bushings.
Semiconductor Processing
In semiconductor fabrication, high-temperature annealing furnaces are used to modify the properties of silicon wafers. The process requires an ultra-pure atmosphere. Platinum heaters are used because they do not outgas or release particles that could create defects on the wafer.
Understanding the Trade-offs
Despite its superior performance, platinum is not a universal solution. Its selection comes with significant compromises that must be carefully considered.
The Prohibitive Cost
Platinum is a precious metal, and its price is the single greatest barrier to its widespread use. Its cost can be hundreds or even thousands of times higher than that of conventional heating element alloys.
This economic reality restricts its use to only the most demanding applications where no other material can provide the required performance and longevity.
Mechanical Strength at High Temperatures
While chemically robust, platinum becomes very soft and mechanically weak as it approaches its upper temperature limits. Platinum heating elements often require structural support from high-purity ceramic tubes or holders to prevent sagging and deformation over time.
For this reason, it is often alloyed with rhodium (another platinum-group metal) to increase its hot strength and creep resistance, albeit at an even higher cost.
Making the Right Choice for Your Application
The decision to use platinum must be justified by a clear, mission-critical need. The choice hinges on your primary goal.
- If your primary focus is reliability and purity at extreme temperatures (above 1200°C): Platinum is often the only viable choice, as its chemical inertness and stable performance are unmatched.
- If your primary focus is cost-effective heating for standard applications (below 1200°C): Materials like Kanthal (FeCrAl) or Nichrome (NiCr) are far more practical and economical solutions.
Ultimately, selecting platinum is an engineering decision to prioritize process integrity and operational longevity over initial equipment cost.
Summary Table:
| Property | Benefit | Application |
|---|---|---|
| High melting point (1768.3°C) | Enables operation up to 1300°C in air | High-temperature furnaces for labs and industry |
| Chemical inertness | Resists corrosion and oxidation, ensuring purity | Semiconductor processing and glass manufacturing |
| Stable electrical resistance | Allows precise temperature control | Analytical instruments like TGA and DSC |
Need a high-temperature furnace solution that ensures purity and reliability? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere, and CVD/PECVD Systems. With strong deep customization capabilities, we precisely meet the unique experimental requirements of diverse laboratories. Contact us today to discuss how our platinum-based heating elements can enhance your processes!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What are the properties and applications of silicon carbide (SiC)? Unlock High-Temperature Performance
- Why are silicon carbide heating elements essential in high-temperature industries? Unlock Reliable, Extreme Heat Solutions
- What makes silicon carbide heating elements resistant to chemical corrosion? Discover the Protective Oxide Layer
- What are the advantages of using high purity green silicon carbide powder in heating elements? Boost Efficiency and Lifespan
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights



















