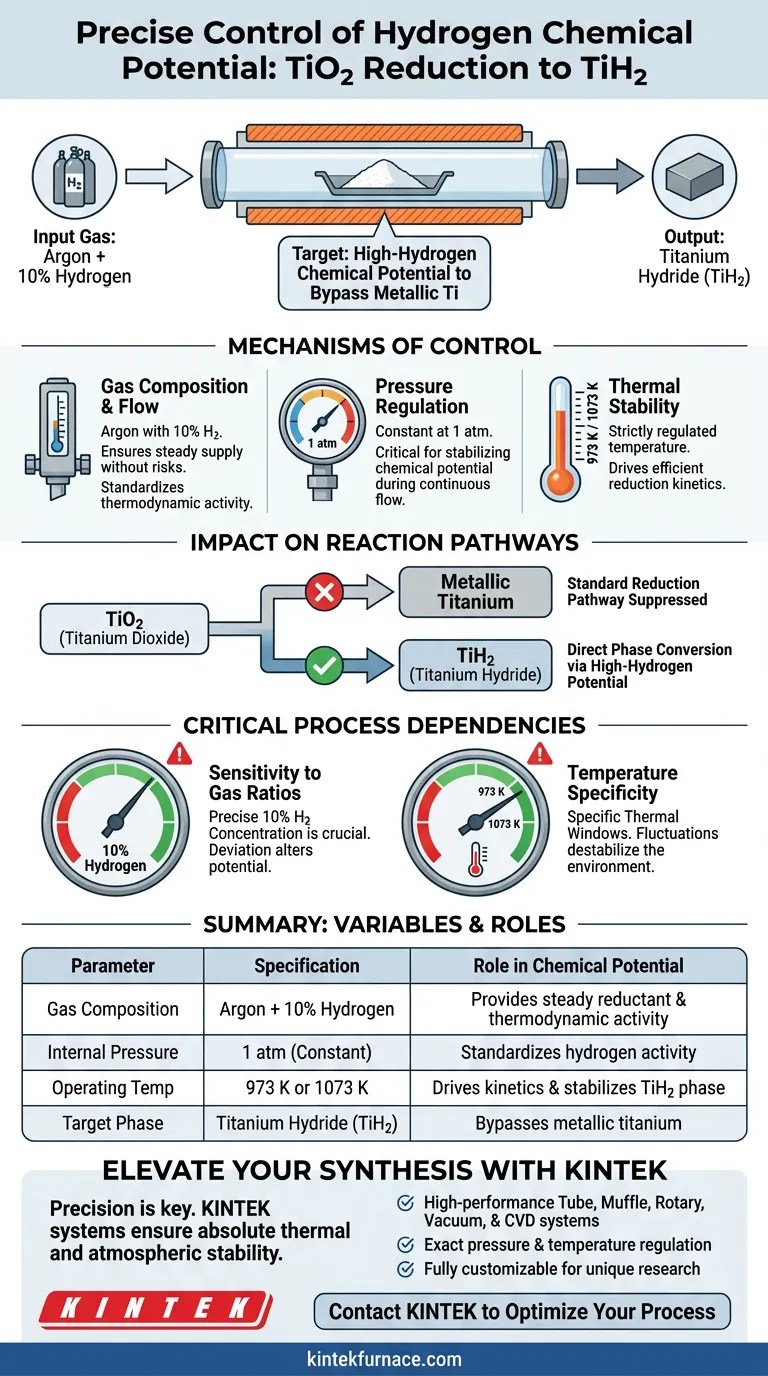
Precise control of the hydrogen chemical potential within a laboratory tube furnace is achieved by continuously introducing a specific gas mixture of Argon and 10% Hydrogen while maintaining an internal pressure of 1 atm. This consistent atmospheric composition, paired with a strictly regulated temperature of either 973 K or 1073 K, creates the exact thermodynamic environment required to dictate the reduction pathway of Titanium Dioxide (TiO2).
The core objective of this configuration is to establish a high-hydrogen chemical potential that alters the standard reduction sequence. This environment forces TiO2 to bypass the metallic titanium stage entirely, converting directly into a stable Titanium Hydride (TiH2) phase.

The Mechanisms of Control
Gas Composition and Flow
The foundation of the process is the introduction of a mixed gas stream. By utilizing Argon combined with 10% Hydrogen, the system ensures a steady supply of the reductant without the safety risks or reaction kinetics associated with pure hydrogen.
Pressure Regulation
Maintaining the internal environment at a constant pressure is critical for stabilizing the chemical potential. The tube furnace is operated strictly at 1 atm, which standardizes the thermodynamic activity of the hydrogen gas during the continuous flow.
Thermal Stability
The chemical potential is also a function of temperature. The furnace’s control system locks the reaction environment at high temperatures, specifically 973 K or 1073 K, to drive the reduction kinetics efficiently.
Impact on Reaction Pathways
Bypassing Metallic Titanium
In standard reduction scenarios, TiO2 might reduce to metallic titanium. However, the specific hydrogen potential created by this setup suppresses that transition.
Direct Phase Conversion
Instead of forming metal, the oxide converts directly into the TiH2 phase. This direct conversion is only possible because the high-hydrogen chemical potential makes the hydride phase thermodynamically favorable over the metallic phase.
Critical Process Dependencies
Sensitivity to Gas Ratios
The success of this process relies heavily on the precise 10% Hydrogen concentration. Deviating from this ratio alters the chemical potential, which could lead to incomplete reduction or the formation of unwanted intermediate phases.
Temperature Specificity
While the process works at 973 K and 1073 K, these are not arbitrary figures. Significant fluctuations outside these specific thermal windows can destabilize the high-hydrogen potential environment, potentially preventing the formation of stable TiH2.
Making the Right Choice for Your Goal
To replicate this reduction process effectively, you must align your furnace parameters with the desired phase outcome.
- If your primary focus is direct hydride formation: Ensure your gas supply maintains a strict 10% Hydrogen balance in Argon to bypass the metallic titanium stage.
- If your primary focus is process stability: Calibrate your furnace to hold a steady 1 atm pressure at exactly 973 K or 1073 K to maintain the necessary chemical potential.
By rigorously controlling these three variables—gas composition, pressure, and temperature—you dictate the thermodynamic rules of the reduction.
Summary Table:
| Parameter | Specification | Role in Chemical Potential |
|---|---|---|
| Gas Composition | Argon + 10% Hydrogen | Provides steady reductant supply and thermodynamic activity |
| Internal Pressure | 1 atm (Constant) | Standardizes hydrogen activity during continuous flow |
| Operating Temp | 973 K or 1073 K | Drives reduction kinetics and stabilizes the TiH2 phase |
| Target Phase | Titanium Hydride (TiH2) | Bypasses metallic titanium via high-hydrogen potential |
Elevate Your Materials Synthesis with KINTEK
Precision is the difference between successful phase conversion and incomplete reduction. At KINTEK, we understand that controlling hydrogen chemical potential requires absolute thermal and atmospheric stability.
Backed by expert R&D and manufacturing, we offer high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems designed to meet your most rigorous lab requirements. Our furnaces provide the exact pressure and temperature regulation needed for sensitive TiO2 reduction and hydride formation, and every system is fully customizable to your unique research needs.
Ready to optimize your reduction process? Contact KINTEK today to consult with our experts and find the perfect high-temperature solution for your laboratory.
Visual Guide
References
- Sung-Hun Park, Jungshin Kang. Direct TiH2 powder production by the reduction of TiO2 using Mg in Ar and H2 mixed gas atmosphere. DOI: 10.1038/s41598-024-84433-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How is heat transferred to the material inside a tube furnace? Master the 3-Stage Process for Precise Thermal Control
- What is the function of autoclaves and tube reactors in hydrometallurgical leaching? Unlock Refractory Ore Potential
- How does a tube furnace differ from HPHT methods for Fe2B-HS? Compare Diffusion and Structural Integrity
- Why is a high-temperature tube furnace required for the activation of nitro-functionalized catalysts? (ACN Mastery)
- Why use a laboratory tube furnace with argon for low carbon steel annealing? Ensure Oxidation-Free Material Integrity
- How does a horizontal tube furnace control the reaction environment for cherry pit carbonization? High-Precision Guide
- What is a Tube Furnace? Master Precision Heating for Sensitive Materials
- What are the key benefits of using split tube furnaces? Unlock Superior Access and Control for Your Lab



















