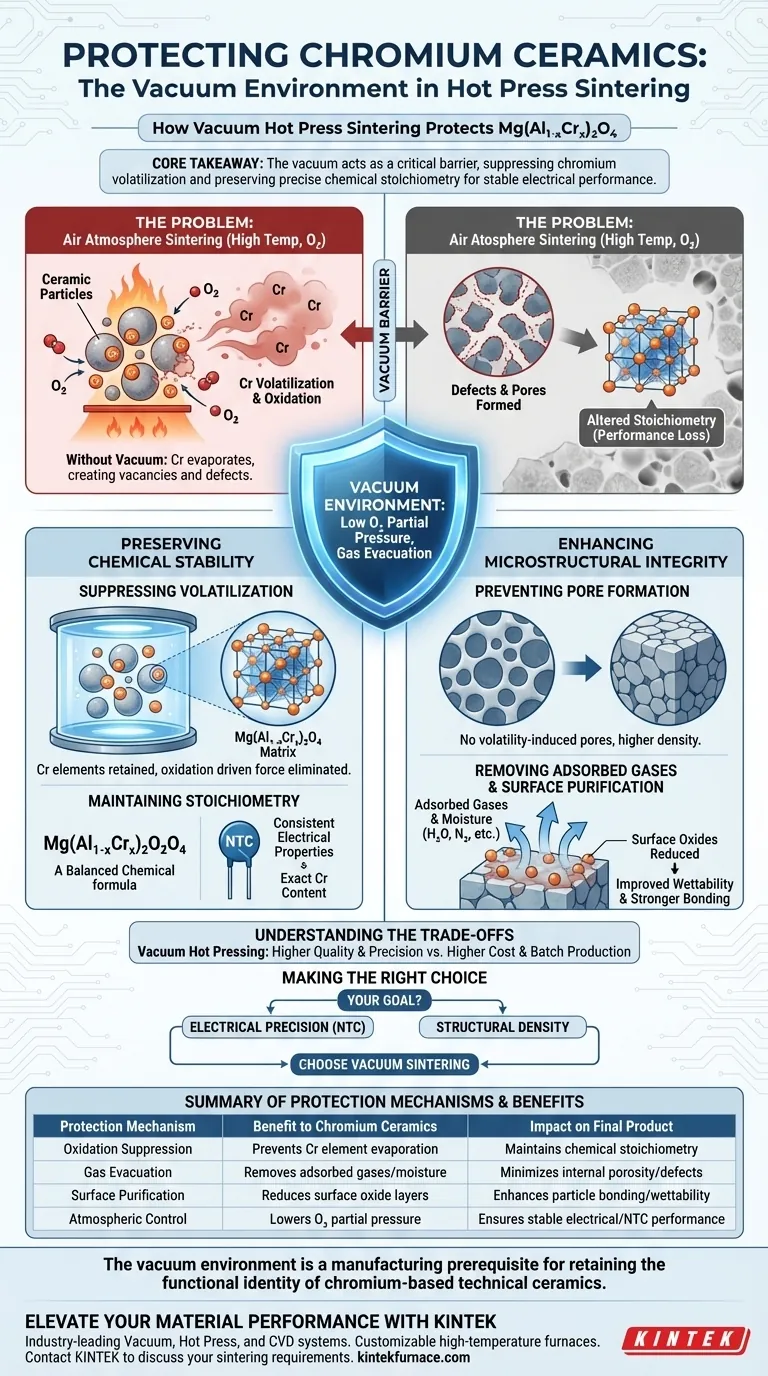
The vacuum environment acts as a critical barrier against chemical degradation for chromium-containing ceramics. Specifically for materials like $Mg(Al_{1-x}Cr_x)_2O_4$, the vacuum suppresses the oxidation and subsequent volatilization of chromium elements that inevitably occur when sintering in air at high temperatures.
Core Takeaway: Sintering chromium-based ceramics in air causes the active chromium to evaporate, altering the material's chemistry and creating defects. The vacuum environment preserves the precise stoichiometric ratio necessary for stable electrical performance and high physical density.

Preserving Chemical Stability
The primary challenge with sintering chromium-containing ceramics is the element's high vapor pressure and reactivity with oxygen.
Suppressing Volatilization
When exposed to high temperatures in an air atmosphere, chromium elements are prone to oxidation. This reaction often leads to volatilization, where the chromium effectively evaporates from the material matrix.
The vacuum environment drastically lowers the oxygen partial pressure. This eliminates the driving force for oxidation, keeping the chromium locked within the solid structure rather than losing it to the atmosphere.
Maintaining Stoichiometry
For complex ceramics like $Mg(Al_{1-x}Cr_x)_2O_4$, the precise ratio of elements (stoichiometry) dictates performance. Losing chromium changes the value of x in the chemical formula.
This shift is not just cosmetic; it fundamentally alters the material's electrical properties. By preventing this loss, the vacuum ensures the final product functions correctly as an NTC (Negative Temperature Coefficient) thermistor.
Enhancing Microstructural Integrity
Beyond chemical protection, the vacuum environment plays a mechanical role in creating a robust ceramic body.
Preventing Pore Formation
When chromium volatilizes in an air atmosphere, it leaves behind vacancies in the crystal lattice. These vacancies coalesce to form pores, resulting in a lower-density, weaker material.
By retaining the chromium, the vacuum environment prevents the generation of these volatility-induced pores.
Removing Adsorbed Gases
Raw ceramic powders naturally hold onto adsorbed gases and moisture. During the heating process, these gases expand and can become trapped, forming closed pores that weaken the material.
The vacuum environment actively evacuates these gases before the material densifies. This allows for a cleaner sintering process and higher final density.
Surface Purification
The vacuum aids in removing volatile impurities and reducing surface oxide layers on the powder particles.
Clean particle surfaces have higher surface energy. This improves wettability, allowing particles to bond more strongly during the diffusion process, further enhancing the material's strength.
Understanding the Trade-offs
While vacuum hot pressing is superior for quality, it introduces specific constraints that must be managed.
Equipment Complexity and Cost
Vacuum hot press systems are significantly more complex than standard air kilns. They require sophisticated pumps, seals, and pressure controls, leading to higher capital and maintenance costs.
Production Throughput
This process is typically a batch operation. Unlike continuous air sintering, vacuum hot pressing restricts the volume of parts produced per hour, making it less suitable for low-cost, mass-market commodities where extreme precision is not required.
Making the Right Choice for Your Goal
To determine if this process aligns with your specific manufacturing needs, consider the following:
- If your primary focus is Electrical Precision: You must use vacuum sintering to maintain the exact chromium content required for consistent thermistor performance.
- If your primary focus is Structural Density: The vacuum is essential for removing adsorbed gases and preventing pore formation, ensuring high mechanical strength.
The vacuum environment is not merely a protective measure; it is a manufacturing prerequisite for retaining the functional identity of chromium-based technical ceramics.
Summary Table:
| Protection Mechanism | Benefit to Chromium Ceramics | Impact on Final Product |
|---|---|---|
| Oxidation Suppression | Prevents Cr element evaporation/volatilization | Maintains precise chemical stoichiometry |
| Gas Evacuation | Removes adsorbed gases and moisture | Minimizes internal porosity and defects |
| Surface Purification | Reduces surface oxide layers on powder | Enhances particle bonding and wettability |
| Atmospheric Control | Lowers oxygen partial pressure | Ensures stable electrical/NTC performance |
Elevate Your Material Performance with KINTEK
Maintaining the precise stoichiometry of chromium-based ceramics requires expert-level atmospheric control. KINTEK provides industry-leading Vacuum, Hot Press, and CVD systems designed to eliminate volatilization and maximize structural density.
Backed by expert R&D and specialized manufacturing, our high-temperature furnaces are fully customizable to meet your unique research or production needs. Don't compromise on electrical precision or mechanical strength.
Contact KINTEK today to discuss your sintering requirements and see how our advanced thermal solutions can optimize your technical ceramic production.
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- Why is secondary processing using a hydraulic press and a sintering furnace necessary for aluminum matrix composites?
- Why is a medium frequency induction heating system utilized in the manufacture of diamond drills via vacuum hot pressing? For Superior Speed and Durability
- What is the function of high-purity graphite molds in FAST? The Key to Precision Sintering Performance
- What physical conditions are provided by the heating plate and high-voltage DC power supply? Mastery of Anodic Bonding
- How does the application of mechanical pressure contribute to the vacuum hot pressing formation of TiAl/Ti6Al4V? Expert Analysis
- What capabilities do vacuum hot press furnaces offer for material manufacturing and processing? Unlock High-Density, Pure Materials
- How do pressure parameters in a vacuum hot press influence stainless steel? Master High-Performance Densification
- What is the core function of a vacuum hot press furnace? Achieve Near-Perfect Densification for Nano-Copper



















