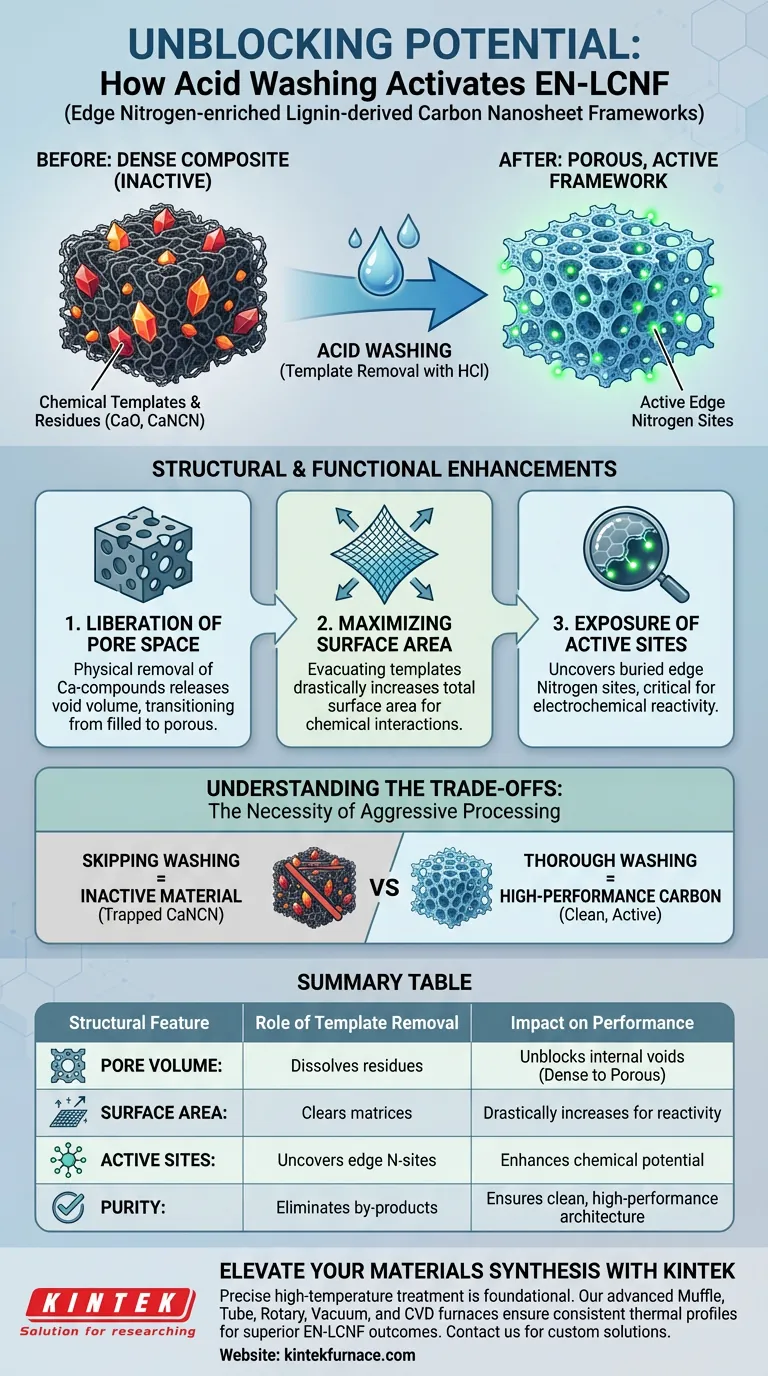
The removal of chemical templates via acid washing acts as a critical activation step, transforming a dense composite into a highly porous, functional framework. By dissolving residual inorganic compounds, this process unblocks internal voids and significantly expands the material's specific surface area and chemical reactivity.
Post-treatment acid washing is not merely a cleaning step; it is a structural modification process. It eliminates solid impurities to liberate pore volume and expose critical edge nitrogen sites, directly determining the material's final performance capabilities.
The Mechanism of Template Extraction
Dissolution of Inorganic Residues
Following high-temperature furnace treatment, the carbon framework remains clogged with chemical templates and reaction by-products.
To address this, the product undergoes washing with an industrial-grade hydrochloric acid solution.
This chemical bath targets and dissolves specific impurities, including calcium oxide, residual calcium carbonate, and calcium cyanamide (CaNCN).
Clearing Internal and Surface Matrices
The acid treatment acts comprehensively on the material structure.
It removes impurities from both the internal and surface areas of the carbon framework.
This ensures that the carbon architecture is cleared of blocking mineral phases that would otherwise impede performance.
Structural and Functional Enhancements
Liberation of Pore Space
The physical removal of calcium-based compounds leaves behind voids where the solids previously resided.
This process effectively releases the pore space that was occupied by the templates during synthesis.
As a result, the material transitions from a filled, dense composite into an open, porous structure.
Maximizing Specific Surface Area
The most immediate physical outcome of this unblocking process is a significant increase in the specific surface area.
By evacuating the template material, the total surface area available for chemical interactions increases drastically.
This expansion is essential for applications requiring high interfacial contact, such as energy storage or catalysis.
Exposure of Active Sites
Beyond physical porosity, the washing process uncovers the material's chemical potential.
It exposes active edge nitrogen sites that were previously masked or buried by the calcium by-products.
These nitrogen sites are critical for the material's reactivity, serving as the primary active centers for electrochemical processes.
Understanding the Trade-offs
The Necessity of Aggressive Processing
While high-temperature treatment creates the carbon backbone, it inevitably leaves the material in an inactive state due to pore blockage.
Skipping or shortening the acid washing step is a common pitfall that leaves calcium cyanamide (CaNCN) and other residues trapped within the matrix.
This results in a material with low surface area and covered active sites, essentially negating the benefits of the edge nitrogen doping.
Optimizing Material Synthesis
To ensure the highest quality EN-LCNF material, the post-processing phase must be treated with the same precision as the initial heating.
- If your primary focus is Physical Porosity: Ensure the hydrochloric acid wash is thorough enough to dissolve all internal calcium carbonate and oxide to maximize void volume.
- If your primary focus is Chemical Reactivity: Prioritize the complete removal of surface impurities to fully uncover the active edge nitrogen sites.
The efficacy of the final carbon framework is defined not just by how it is built, but by how effectively it is cleaned.
Summary Table:
| Structural Feature | Role of Template Removal (Acid Washing) | Impact on Performance |
|---|---|---|
| Pore Volume | Dissolves CaO and CaNCN residues | Unblocks internal voids; transitions from dense to porous |
| Surface Area | Clears surface and internal matrices | Drastically increases specific surface area for reactivity |
| Active Sites | Uncovers buried edge nitrogen sites | Enhances chemical potential and electrochemical activity |
| Purity | Eliminates mineral phases and by-products | Ensures a clean, high-performance carbon architecture |
Elevate Your Materials Synthesis with KINTEK
Precise high-temperature treatment is the foundation of high-performance carbon frameworks. KINTEK provides the advanced heating technology required to drive complex chemical template reactions with unmatched accuracy. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the unique structural requirements of your research.
Whether you are developing EN-LCNF for energy storage or catalysis, our lab high-temp furnaces ensure consistent thermal profiles for superior material outcomes. Contact us today to find your custom furnace solution and maximize your lab's productivity.
Visual Guide
References
- Caiwei Wang, Zhili Li. Engineering of edge nitrogen dopant in carbon nanosheet framework for fast and stable potassium-ion storage. DOI: 10.1007/s44246-024-00101-8
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the three main categories of thin-film deposition methods? Essential Guide for Material Engineers
- How does a programmable high-temperature furnace improve the control of cooling rates? Enhance Ceramic Part Integrity
- What role does a laboratory facility play in establishing the mass balance for a coke oven operation? Drive Efficiency.
- How does the combination of a nitrogen atmosphere and magnetic stirring benefit the dissolution stage? | KINTEK
- What is the role of a laboratory oven in the pretreatment of raw materials? Optimize EBC Powder Flowability
- What are the technological advantages of using a Rapid Thermal Annealing (RTA) system? Precision for Semiconductors
- How does the single-stage artificial aging process (T6 state) strengthen AA7050 aluminum alloy wire?
- What is the function of a laboratory vacuum drying oven for Fe-N-C catalysts? Preserve Nanoporous Structure



















