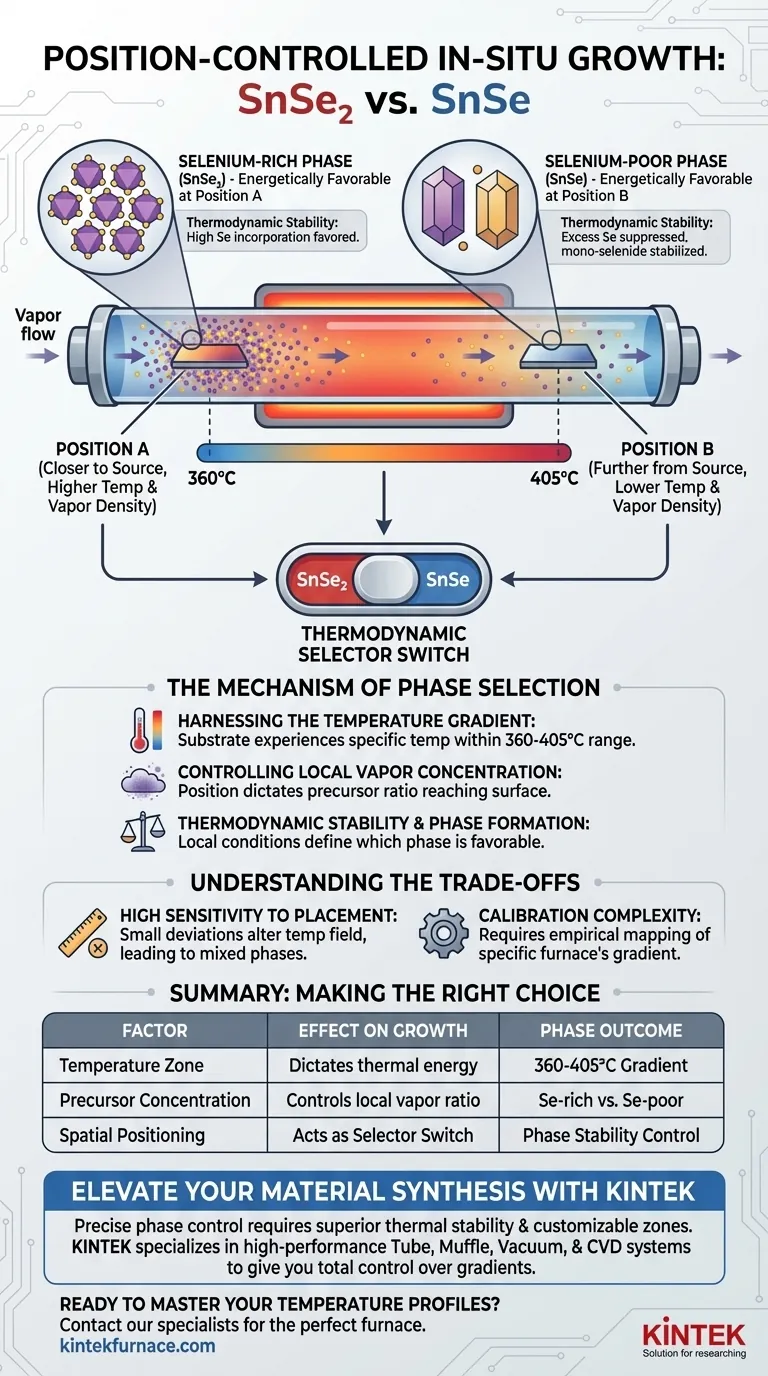
The positioning of the substrate is the decisive factor in controlling the phase composition of Tin Selenide during in-situ growth. By placing the substrate at specific distances from the heating center, you expose it to a unique temperature field and local precursor concentration. This precise placement allows you to selectively grow either Selenium-rich (SnSe2) or Selenium-poor (SnSe) phases within the same experimental setup.
Core Takeaway The substrate’s physical location acts as a thermodynamic selector switch. By utilizing the furnace's natural temperature gradient, shifting the substrate allows you to toggle between growing SnSe2 and SnSe by altering the local thermal energy and vapor ratio without changing the external source settings.

The Mechanism of Phase Selection
Harnessing the Temperature Gradient
A tube furnace does not maintain a uniform temperature throughout its entire length.
There is a natural temperature gradient, typically ranging from 360 to 405 degrees Celsius in this context.
The specific position of the substrate determines the exact temperature it experiences within this range.
Controlling Local Vapor Concentration
Positioning dictates more than just surface temperature.
The location affects the local concentration ratio of the precursor vapors reaching the substrate surface.
As vapors travel away from the source, their density and mixing ratios evolve, creating distinct chemical environments at different distances.
Thermodynamic Stability and Phase Formation
The combination of local temperature and vapor concentration creates specific thermodynamic conditions.
These conditions dictate which crystalline phase is energetically favorable to form at that exact spot.
One position provides the stability required for the Selenium-rich phase (SnSe2), while a different position favors the Selenium-poor phase (SnSe).
Understanding the Trade-offs
High Sensitivity to Placement
The reliance on a spatial gradient means the process is extremely sensitive to physical positioning.
A deviation of just a few centimeters can drastically alter the temperature field the substrate experiences.
This can lead to unintentional mixed-phase growth if the substrate spans a transition zone between the two thermodynamic stability regions.
Calibration Complexity
Relying on the natural gradient requires precise mapping of your specific furnace.
The 360 to 405 degrees Celsius range is a general operating window, but the exact profile can vary between equipment.
You must empirically determine the exact "sweet spot" distances for pure phase growth in your specific hardware.
Making the Right Choice for Your Goal
To effectively utilize position-controlled growth, you must treat the furnace tube as a coordinate system where distance equals chemical composition.
- If your primary focus is the Selenium-rich phase (SnSe2): Calibrate your substrate placement to find the specific zone in the gradient where thermodynamic stability supports high selenium incorporation.
- If your primary focus is the Selenium-poor phase (SnSe): Shift the substrate to the distance where the temperature and concentration ratio suppress excess selenium, stabilizing the mono-selenide structure.
Mastering the spatial profile of your furnace allows you to dictate material properties simply by moving your sample.
Summary Table:
| Factor | Effect on Growth | Phase Outcome |
|---|---|---|
| Temperature Zone | Dictates thermal energy for reaction | 360-405°C Gradient |
| Precursor Concentration | Controls local vapor density/mixing ratio | Se-rich vs. Se-poor |
| Spatial Positioning | Acts as a thermodynamic selector switch | Phase Stability Control |
| Distance from Source | Influences chemical environment evolution | Selective SnSe2 or SnSe |
Elevate Your Material Synthesis with KINTEK
Precise phase control in SnSe2 and SnSe growth requires equipment with superior thermal stability and customizable zones. At KINTEK, we specialize in high-performance Tube, Muffle, Vacuum, and CVD systems designed to give you total control over your experimental gradients.
Whether you need a standard setup or a custom-engineered solution for your unique R&D needs, our expert manufacturing team is ready to support your breakthrough.
Ready to master your temperature profiles? Contact our specialists today to find the perfect furnace for your lab.
Visual Guide
References
- Manab Mandal, K. Sethupathi. In Situ Simultaneous Growth of Layered SnSe<sub>2</sub> and SnSe: a Linear Precursor Approach. DOI: 10.1002/admi.202500239
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- How do the components of a tube furnace contribute to its overall performance? Optimize Your Lab's Heat Processing Efficiency
- What is the primary role of a tube furnace in CuGaO2 treatment? Enhance Crystallization and Film Performance
- How is heat transferred to the material inside a tube furnace? Master the 3-Stage Process for Precise Thermal Control
- Why are atmospheric controls important in horizontal tube furnaces? Ensure Precise Chemical Processing and Safety
- How does the injection probe in a Drop Tube Furnace ensure a high heating rate? Simulate Industrial Pyrolysis Expertly
- Why is high-vacuum encapsulation in quartz tubes required? Ensure Precision for Sn-Ag-Bi-Se-Te Composites
- What is the role of the tubular furnace annealing process in the synthesis of Si@SnO2? Achieve Precise Nanocoatings
- What is the purpose of the gas circulation system in a tube furnace? Control Chemical Atmospheres for Precise High-Temperature Processing



















