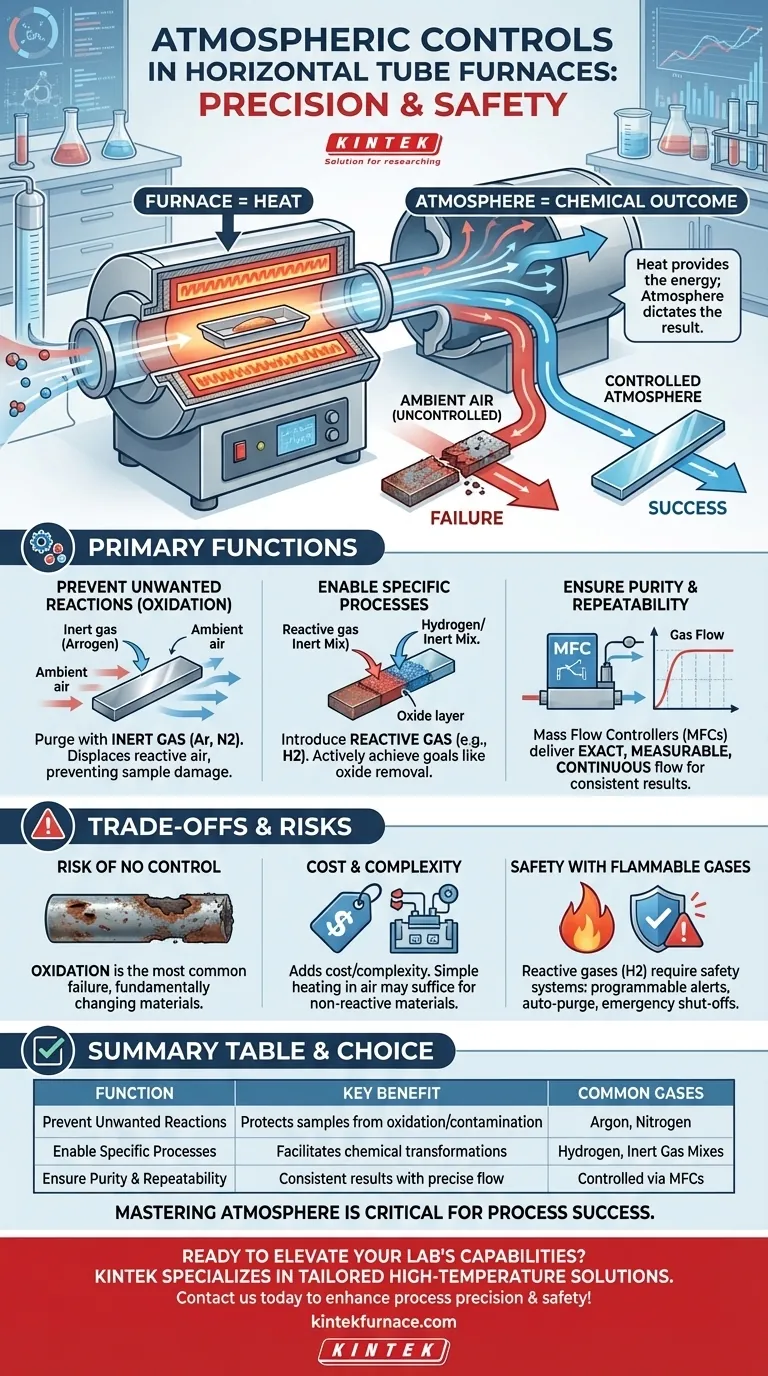
At their core, atmospheric controls are vital in horizontal tube furnaces because they allow you to precisely manage the chemical environment during high-temperature processing. While the furnace provides the necessary heat, the gas atmosphere inside the tube dictates the chemical outcome of your process. This control is critical for preventing unwanted reactions, enabling specific material transformations, and ensuring a safe, repeatable procedure.
A horizontal tube furnace provides heat, but the atmosphere determines the result. Without atmospheric controls, you expose your process to ambient air, leading to oxidation and contamination that can ruin samples, compromise research, and cause production failures.

The Primary Functions of a Controlled Atmosphere
A controlled atmosphere moves your process from simple heating to precise thermal-chemical engineering. Its importance can be broken down into three key functions.
Preventing Unwanted Chemical Reactions
At high temperatures, most materials are highly reactive with the oxygen and moisture present in ambient air. This reaction, oxidation, is often undesirable and can damage or destroy your sample.
Atmospheric controls allow you to purge the furnace tube with an inert gas, such as argon or nitrogen. This displaces the reactive air, creating a neutral environment that protects the material from oxidation and contamination.
Enabling Specific Chemical Processes
Beyond simply protecting a sample, atmospheric controls can create a reactive environment to achieve a specific goal.
For example, introducing a reducing gas like hydrogen (often mixed with an inert gas for safety) can actively remove oxides from a material's surface. This is a common requirement in semiconductor and metallurgy applications.
Ensuring Purity and Repeatability
For any scientific or manufacturing process, repeatability is paramount. Atmospheric controls, particularly those using Mass Flow Controllers (MFCs), provide this consistency.
MFCs act as highly precise digital valves, delivering an exact, measurable, and continuous flow of gas. This ensures that the chemical environment is identical for every run, leading to reliable and predictable results.
Understanding the Trade-offs and Risks
While essential for many applications, implementing atmospheric controls involves considering their complexity and the risks of foregoing them.
The Risk of No Control: Oxidation
The most common failure in processes without atmospheric control is oxidation. For sensitive metals or advanced materials, heating in ambient air will almost certainly form an unwanted oxide layer, fundamentally changing the material's properties and rendering the process a failure.
The Cost and Complexity Factor
Gas delivery systems, mixers, and MFCs add cost and complexity to a furnace setup. For some applications, such as firing stable ceramics that are not sensitive to oxygen, heating in ambient air is perfectly acceptable and more cost-effective. The need for control is entirely dependent on the material and the process goal.
Safety with Flammable Gases
Using reactive gases like hydrogen introduces significant safety risks. Proper atmospheric control systems are designed with this in mind, incorporating features like programmable safety alerts, automated purge cycles, and emergency shut-offs to manage the risks of handling flammable or toxic gases.
Making the Right Choice for Your Goal
Selecting the appropriate level of atmospheric control depends entirely on your material and desired outcome.
- If your primary focus is simple heat treatment of non-reactive materials (e.g., firing certain ceramics): You may not require complex atmospheric controls, as heating in ambient air can be sufficient.
- If your primary focus is preventing oxidation on sensitive materials (e.g., annealing metals): An inert gas delivery system (using argon or nitrogen) is the minimum requirement to protect your sample.
- If your primary focus is active chemical processing (e.g., reducing surface oxides or chemical vapor deposition): A sophisticated system with gas mixing capabilities and precise Mass Flow Controllers is essential for control and repeatability.
Ultimately, mastering your furnace's atmosphere is just as critical as mastering its temperature.
Summary Table:
| Function | Key Benefit | Common Gases Used |
|---|---|---|
| Prevent Unwanted Reactions | Protects samples from oxidation and contamination | Argon, Nitrogen |
| Enable Specific Processes | Facilitates chemical transformations like reduction | Hydrogen, Inert Gas Mixes |
| Ensure Purity and Repeatability | Provides consistent results with precise gas flow | Controlled via Mass Flow Controllers |
Ready to elevate your lab's capabilities with advanced atmospheric control? At KINTEK, we specialize in high-temperature furnace solutions tailored to your needs. Leveraging exceptional R&D and in-house manufacturing, we offer products like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all with deep customization to meet your unique experimental requirements. Contact us today to discuss how our expertise can enhance your process precision and safety!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- What role does a laboratory tube furnace perform during the carbonization of LCNSs? Achieve 83.8% Efficiency
- Why is a tube furnace utilized for the heat treatment of S/C composite cathode materials? Optimize Battery Stability
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis



















