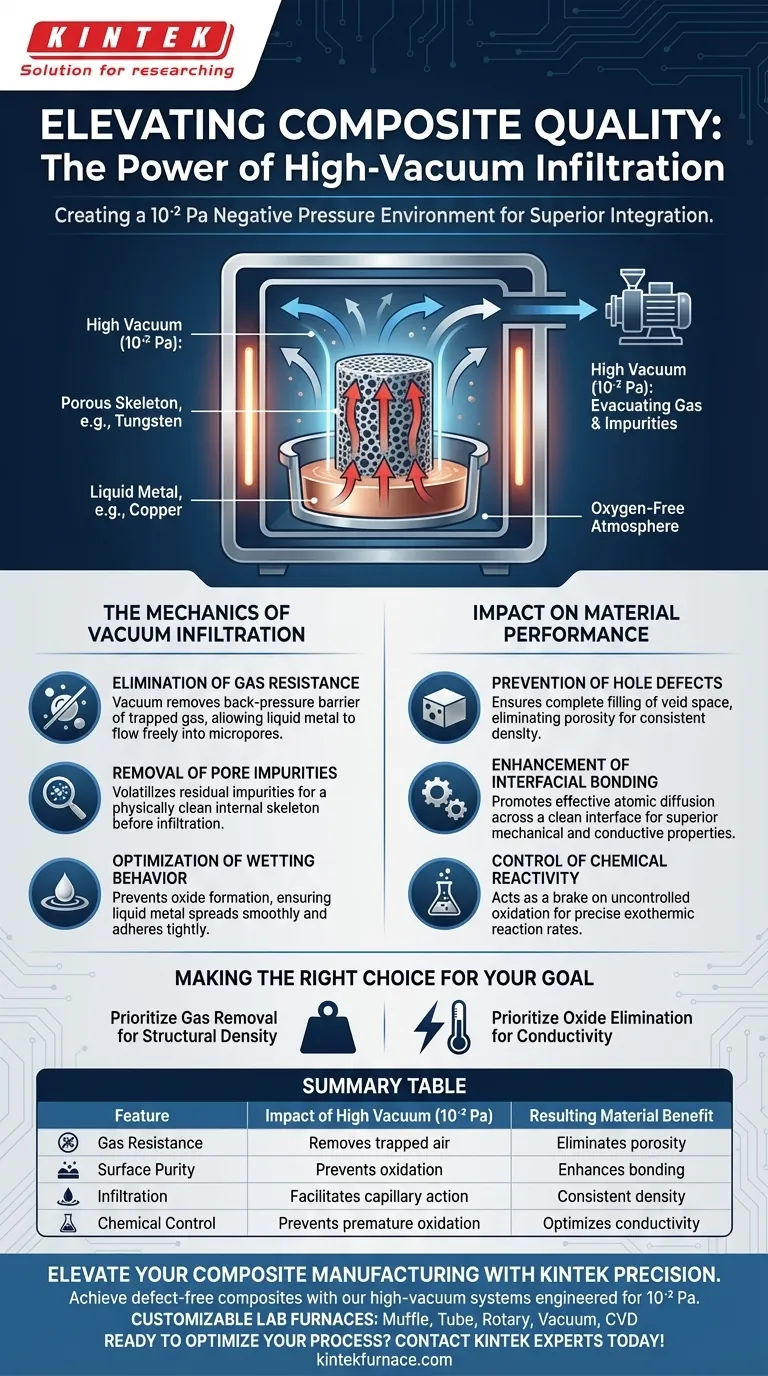
The negative pressure environment created by a high vacuum infiltration furnace functions as a critical purification and enabling mechanism for composite integration.
By maintaining a vacuum level at approximately 10⁻² Pa, the furnace actively evacuates residual gases trapped within the pores of the solid skeleton (such as tungsten). Simultaneously, it creates an oxygen-free atmosphere that prevents impurity formation, ensuring that the liquid metal (such as copper) encounters no physical resistance or chemical barriers during infiltration.
Core Takeaway The vacuum environment is not merely about removing air; it is about eliminating gas resistance and preventing surface oxidation. This dual action ensures liquid metal can completely penetrate micropores via capillary action, resulting in a fully dense composite with superior interfacial bonding and no structural voids.

The Mechanics of Vacuum Infiltration
Elimination of Gas Resistance
In a standard atmospheric environment, the pores of a porous skeleton are filled with gas.
If this gas is not removed, it acts as a back-pressure barrier against the infiltrating liquid metal.
The high vacuum environment removes this gas resistance, allowing the liquid metal to flow freely into the skeletal micropores without fighting against trapped air pockets.
Removal of Pore Impurities
Beyond simple air evacuation, the negative pressure aids in volatilizing and removing residual impurities residing deep within the material's pores.
This ensures that the internal structure of the skeleton is physically clean before the infiltration process begins.
A clean pore structure is a prerequisite for achieving consistent density throughout the composite.
Optimization of Wetting Behavior
For successful infiltration, the liquid metal must "wet" the solid skeleton—meaning it must spread across the surface rather than bead up.
Impurities and oxides on the surface of the solid skeleton significantly degrade this wetting ability.
By providing an oxygen-free environment, the furnace prevents these wetting-inhibitors from forming, allowing the liquid metal to spread smoothly and adhere tightly to the solid structure.
Impact on Material Performance
Prevention of Hole Defects
The primary defect in infiltration processes is porosity—tiny holes left where metal failed to penetrate.
By eliminating gas pockets that would otherwise occupy space, the vacuum ensures complete filling of the void space.
This results in a dense composite material free of the "hole defects" that compromise structural integrity.
Enhancement of Interfacial Bonding
The vacuum environment does more than just fill space; it ensures the quality of the bond between the two materials.
Supplementary data indicates that a clean, oxide-free interface promotes effective atomic diffusion between the matrix (e.g., copper) and the reinforcement (e.g., tungsten or carbides).
This superior bonding directly translates to enhanced mechanical hardness and better electrical or thermal conductivity.
Control of Chemical Reactivity
In reactive systems, such as Reactive Melt Infiltration (RMI), the vacuum acts as a "brake" on uncontrolled oxidation.
It prevents the premature oxidation of active metals (like silicon or zirconium) which ensures the metal remains fluid enough to infiltrate fully.
This precise environmental control allows for the correct exothermic reaction rates needed to form the desired composite phases.
Understanding the Trade-offs
Equipment Complexity and Cost
Achieving and maintaining a vacuum of 10⁻² Pa requires sophisticated pumping systems and robust sealing.
This increases both the initial capital investment and the operational complexity compared to positive-pressure or lower-vacuum alternatives.
Volatilization Risks
While vacuum removes impurities, extremely low pressure at high temperatures can also cause desirable elements to volatilize (evaporate).
Operators must carefully balance temperature and pressure to ensure the liquid metal infiltrates the skeleton rather than evaporating into the furnace chamber.
Making the Right Choice for Your Goal
The level of vacuum you require depends heavily on the sensitivity of your materials to oxidation and the pore size of your preform.
- If your primary focus is Structural Density: Prioritize the removal of gas resistance to eliminate porosity and hole defects, ensuring the liquid metal fills every micropore.
- If your primary focus is Conductivity (Thermal/Electrical): Prioritize the elimination of oxides to ensure a pristine interface between the matrix and the skeleton, as oxides are thermal and electrical insulators.
Ultimately, the high vacuum environment transforms infiltration from a mechanical filling process into a precise chemical bonding event, guaranteeing the density and performance of the final composite.
Summary Table:
| Feature | Impact of High Vacuum (10⁻² Pa) | Resulting Material Benefit |
|---|---|---|
| Gas Resistance | Removes trapped air from skeletal micropores | Eliminates porosity and hole defects |
| Surface Purity | Prevents oxidation and volatilizes impurities | Enhances wetting and interfacial bonding |
| Infiltration | Facilitates capillary action without back-pressure | Ensures consistent density and structural integrity |
| Chemical Control | Prevents premature oxidation of active metals | Optimizes electrical and thermal conductivity |
Elevate Your Composite Manufacturing with KINTEK Precision
Don't let porosity or oxidation compromise your material performance. KINTEK’s high-vacuum infiltration systems are engineered to provide the precise negative pressure environments (down to 10⁻² Pa) required for superior interfacial bonding and maximum density.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab high-temp furnaces—including Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique materials science challenges. Whether you are working with tungsten-copper alloys or reactive melt infiltration, our technical team is ready to help you optimize your process.
Ready to achieve defect-free composites? Contact our experts today to discuss your custom furnace requirements.
Visual Guide
References
- Tan Liu, Yi Ding. Graphene-Enhanced CuW Composites for High-Voltage Circuit Breaker Electrical Contacts. DOI: 10.3390/app14072731
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the temperature of a vacuum furnace? Achieve High-Purity Thermal Processing
- What are the main advantages of customized vacuum furnaces? Unlock Tailored Precision for Superior Quality
- What are vacuum furnaces used for? Achieve Unmatched Material Purity and Performance
- What is the role of vacuum tempering furnaces? Enhance Material Toughness and Surface Quality
- What is the primary function of a vacuum drying oven in the synthesis of H2bdt organic ligands? Protect Your Purity.
- What role does a high-vacuum or low-oxygen furnace play in NiCoCrAlY pre-oxidation? Master Selective Oxidation Control
- What advantages do vacuum and modified atmosphere furnaces offer for sintering in 3D printing? Achieve Dense, High-Performance Parts
- How can vacuum heat treatment improve the service life of mechanical parts and tools? Boost Durability and Extend Lifespan



















