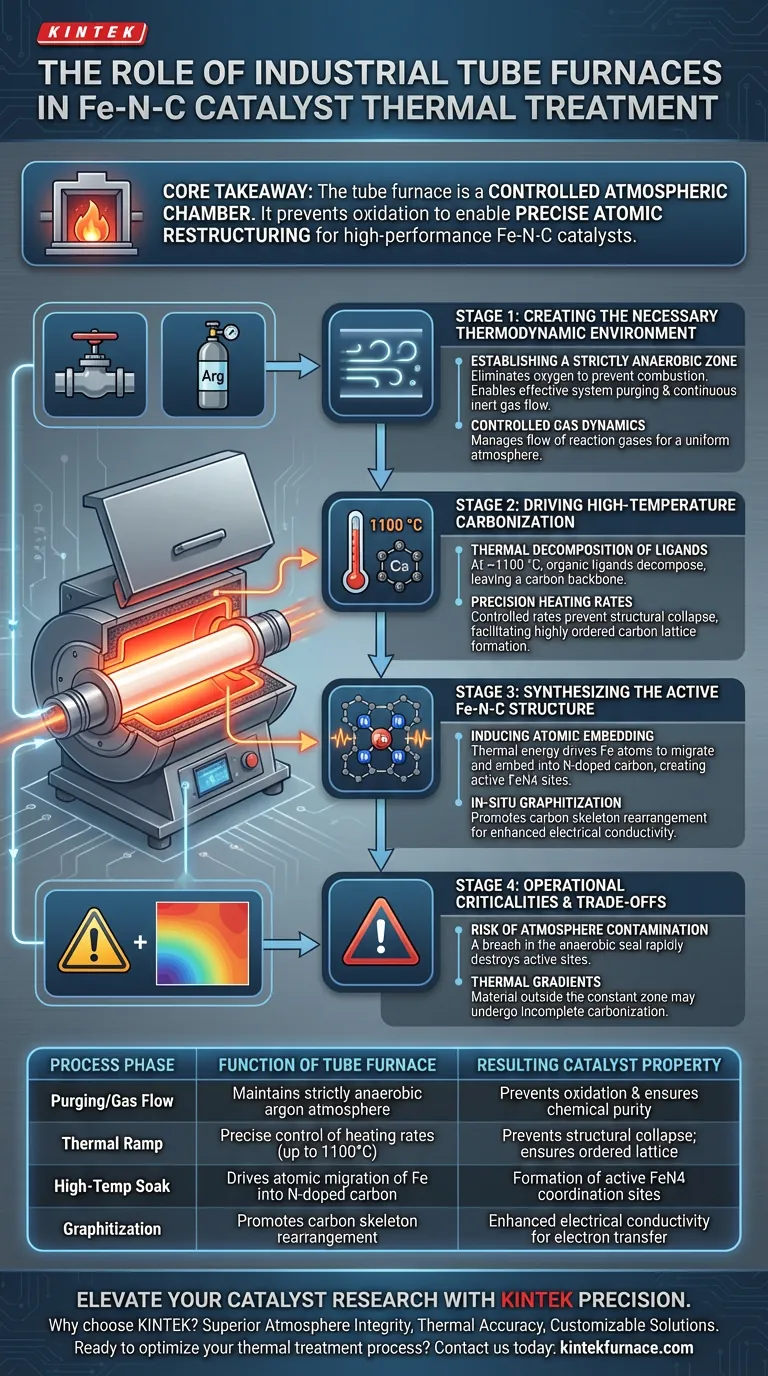
The industrial tube furnace serves as the foundational reactor for synthesizing Fe-N-C catalysts by providing a strictly anaerobic environment essential for chemical transformation. By maintaining a stable flow of inert gas (typically argon) at high temperatures around 1100 °C, the furnace drives the carbonization of organic frameworks and forces iron atoms to embed into the nitrogen-doped carbon substrate, resulting in the formation of highly active FeN4 structures.
Core Takeaway The tube furnace is not merely a heating source; it is a controlled atmospheric chamber that prevents oxidation, enabling the precise atomic restructuring required to turn raw precursors into stable, high-performance Fe-N-C catalysts.

Creating the Necessary Thermodynamic Environment
Establishing a Strictly Anaerobic Zone
The primary contribution of the tube furnace is the elimination of oxygen.
Fe-N-C synthesis requires the carbonization of precursors, a process that would fail if oxygen were present, leading to combustion rather than graphitization.
The tube design allows for effective system purging and the maintenance of a continuous inert gas flow, such as argon.
Controlled Gas Dynamics
Beyond simple exclusion of air, the furnace manages the flow of reaction gases.
By allowing the sequential introduction of inert or reducing gases, the equipment creates a uniform atmosphere throughout the heating zone.
This uniformity ensures that every part of the precursor material experiences the same chemical environment, preventing inconsistent catalytic properties.
Driving High-Temperature Carbonization
Thermal Decomposition of Ligands
The furnace operates at specific high-temperature plateaus, often cited at 1100 °C for this specific catalyst class.
At these temperatures, the organic ligands within the precursor undergo complete thermal decomposition.
This effectively strips away non-essential elements, leaving behind the carbon backbone necessary for the catalyst's structure.
Precision Heating Rates
The ability to control the rate of temperature increase is as critical as the maximum temperature itself.
Precise heating rates ensure that the decomposition happens in a controlled manner, preventing the structural collapse of the material.
This control facilitates the formation of a highly ordered carbon lattice, which is the physical skeleton of the final catalyst.
Synthesizing the Active Fe-N-C Structure
Inducing Atomic Embedding
The defining feature of an Fe-N-C catalyst is the specific coordination of iron and nitrogen.
The thermal energy provided by the furnace induces iron atoms to migrate and embed themselves into the nitrogen-doped carbon substrate.
This process creates the FeN4 moieties (an iron atom coordinated with four nitrogen atoms), which are the active sites responsible for the catalyst's performance.
In-Situ Graphitization
The high-temperature environment promotes the graphitization of the carbon skeleton.
This structural rearrangement enhances the electrical conductivity of the material.
Superior conductivity is essential for the catalyst to facilitate electron transfer during electrochemical reactions.
Operational Criticalities and Trade-offs
The Risk of Atmosphere Contamination
The most significant risk in using a tube furnace for this application is a breach in the anaerobic seal.
Even trace amounts of oxygen entering the tube at 1100 °C can lead to the rapid oxidation of the carbon support or the iron species.
This would destroy the active FeN4 sites and produce inactive metal oxides instead.
Thermal Gradients
While tube furnaces offer precise control, thermal gradients can exist towards the ends of the tube.
Material placed outside the constant temperature zone may undergo incomplete carbonization.
Operators must ensure precursors are positioned strictly within the uniform thermal field to guarantee batch consistency.
Making the Right Choice for Your Goal
To maximize the efficacy of your thermal treatment, align your operational parameters with your specific catalytic requirements:
- If your primary focus is maximizing active site density: Prioritize the stability of the argon flow and the purity of the anaerobic environment to protect the FeN4 coordination.
- If your primary focus is structural durability: Focus on the precision of the heating ramp rate to ensure a highly ordered, graphitized carbon lattice without structural collapse.
Success in Fe-N-C synthesis relies less on maximum heat and more on the absolute purity of the reaction atmosphere.
Summary Table:
| Process Phase | Function of Tube Furnace | Resulting Catalyst Property |
|---|---|---|
| Purging/Gas Flow | Maintains strictly anaerobic argon atmosphere | Prevents oxidation and ensures chemical purity |
| Thermal Ramp | Precise control of heating rates (up to 1100°C) | Prevents structural collapse; ensures ordered lattice |
| High-Temp Soak | Drives atomic migration of Fe into N-doped carbon | Formation of active FeN4 coordination sites |
| Graphitization | Promotes carbon skeleton rearrangement | Enhanced electrical conductivity for electron transfer |
Elevate Your Catalyst Research with KINTEK Precision
Precise atmospheric control and thermal uniformity are non-negotiable for successful Fe-N-C synthesis. At KINTEK, we understand that even trace oxygen can compromise your results. Backed by expert R&D and world-class manufacturing, we provide high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of material science.
Why choose KINTEK?
- Superior Atmosphere Integrity: Advanced sealing technologies for strictly anaerobic environments.
- Thermal Accuracy: Uniform heating zones to eliminate gradients and ensure batch consistency.
- Customizable Solutions: Our furnaces are tailored to your unique laboratory or industrial needs.
Ready to optimize your thermal treatment process? Contact us today to discuss your project requirements!
Visual Guide
References
- Yumei Liu, Quanquan Pang. Integrated energy storage and CO2 conversion using an aqueous battery with tamed asymmetric reactions. DOI: 10.1038/s41467-023-44283-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the main uses of tube furnaces in laboratories? Unlock Precision in Material Synthesis and Heat Treatment
- How does the placement of materials within an atmospheric tube furnace affect the yield and purity of LiFePO4?
- What are the technical advantages of using a vacuum tube furnace for S53P4-NO2 glass? Achieve 100% Amorphous Results
- What are the key benefits of using split tube furnaces? Unlock Superior Access and Control for Your Lab
- How does the sealing mechanism in Quartz Tube Furnaces differ from traditional systems? Discover Advanced Sealing for Reliable Results
- Why is a heating rate of 3 °C/min typically set for a tube furnace? Optimize Iron Titanate Catalyst Synthesis
- What is the key component of a tube furnace and how is it constructed? Unlock Precision Heating for Your Lab
- How are tubular furnaces used in heat treatment processes? Unlock Precision in Material Science



















