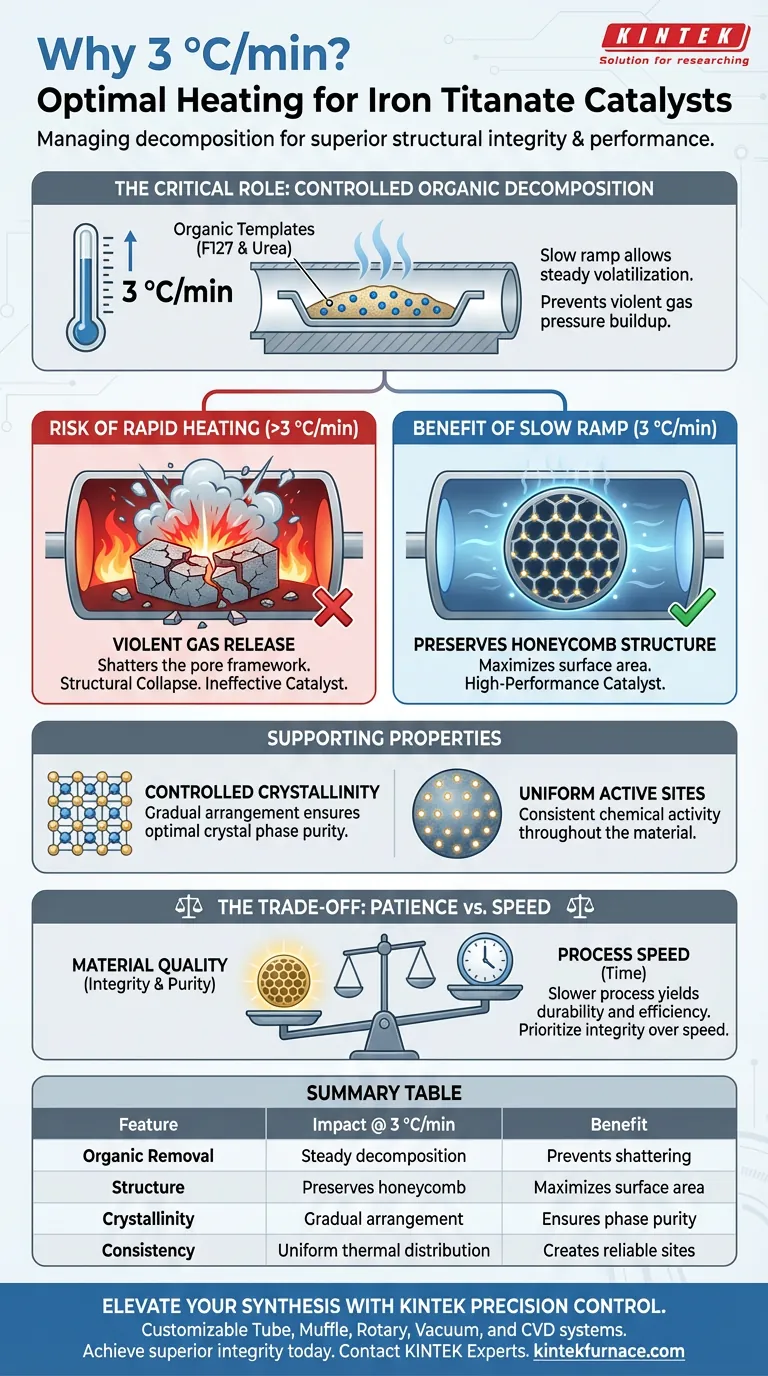
A heating rate of 3 °C/min is chosen specifically to manage the decomposition of organic template agents, such as F127 and urea, used in the synthesis process. This slow, steady ramp prevents the violent release of gases that occurs during rapid heating, ensuring the delicate mesoporous honeycomb structure of the iron titanate remains intact.
Precise thermal control is the mechanism that separates a high-performance catalyst from a collapsed material. By limiting the heating rate, you prioritize the structural integrity of the pore framework over processing speed, ensuring consistent chemical activity.
The Critical Role of Thermal Control
Managing Organic Decomposition
In the preparation of iron titanate catalysts, organic agents like F127 and urea act as templates to shape the material. These substances must be removed to activate the catalyst, but they must be removed gently.
A rate of 3 °C/min allows these organics to decompose and volatilize in a steady, controlled manner. This prevents the sudden pressure buildup that occurs when solid organics flash into gas too quickly.
Protecting the Honeycomb Framework
The primary risk of rapid heating is the destruction of the pore structure. If the organic templates exit the material violently, they can shatter the surrounding architecture.
By keeping the rate low, you preserve the integrity of the mesoporous honeycomb framework. This specific structure is vital because it maximizes the surface area available for catalytic reactions.
Crystalline and Chemical Properties
Achieving Controlled Crystallinity
Beyond porosity, the heating rate dictates how the atomic lattice of the iron titanate forms. A slow ramp provides the thermal energy necessary for atoms to arrange themselves correctly without thermal shock.
This results in controlled crystallinity, ensuring the final material has the specific crystal phase required for optimal performance.
Uniform Active Sites
While the primary reference focuses on pore structure, the general principle of tube furnace operation reinforces the need for uniformity. Precise thermal management ensures that chemical changes occur evenly throughout the material.
Just as with activated carbon or supported metal precursors, a controlled environment allows for the gradient adjustment of chemical properties without damaging the physical support.
Understanding the Trade-offs
Process Efficiency vs. Material Quality
The most significant trade-off with a 3 °C/min heating rate is time. This is a slow process that extends the total duration of the synthesis significantly.
However, in catalyst preparation, efficiency in the furnace often leads to failure in the reactor. Accelerating this step risks collapsing the pores, which renders the catalyst ineffective regardless of how quickly it was produced.
Sensitivity to Precursors
It is important to note that this rate is specific to the volatile nature of the agents used (urea/F127). Changing the template agent might allow for faster rates, or require even slower ones.
The 3 °C/min standard is a calculated balance, optimized specifically to handle the gas release volume of these specific organic templates.
Making the Right Choice for Your Goal
- If your primary focus is Maximum Surface Area: Adhere strictly to the 3 °C/min rate to prevent the collapse of the mesoporous honeycomb structure.
- If your primary focus is Phase Purity: Maintain the slow ramp to allow for the ordered arrangement of the crystal lattice and controlled crystallinity.
- If your primary focus is Process Speed: Acknowledge that increasing the rate significantly increases the risk of structural defects and lowered catalytic activity.
Ultimately, the patience invested during the thermal ramp phase is the deciding factor in the structural durability and efficiency of the final catalyst.
Summary Table:
| Feature | Impact of 3 °C/min Rate | Benefit to Catalyst |
|---|---|---|
| Organic Removal | Steady decomposition of F127 & Urea | Prevents gas buildup & material shattering |
| Structure | Preserves mesoporous honeycomb framework | Maximizes surface area for reactions |
| Crystallinity | Gradual atomic lattice arrangement | Ensures optimal crystal phase purity |
| Consistency | Uniform thermal distribution | Creates reliable, high-activity active sites |
Elevate Your Material Synthesis with Precision Control
Don't let structural collapse undermine your research. KINTEK provides industry-leading thermal solutions backed by expert R&D and manufacturing. Our high-precision Tube, Muffle, Rotary, Vacuum, and CVD systems are fully customizable to meet the exacting heating rates required for delicate catalyst preparation.
Achieve superior crystallinity and structural integrity today.
Visual Guide
References
- Moses D. Ashie, Bishnu Prasad Bastakoti. Photocatalytic Hydrogen Evolution Using Mesoporous Honeycomb Iron Titanate. DOI: 10.1002/smll.202310927
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role does a high-temperature tube furnace play in the solid-state synthesis of LIB cathode materials? Key Insights
- What role does a tube furnace play in the high-temperature heat treatment of vermiculite? Precision Control Expert
- What factors should be considered when purchasing a quartz tube furnace? Ensure Reliable High-Temperature Processing
- How do three-zone tube furnaces contribute to energy and resource efficiency? Boost Lab Performance with Precision Heating
- What conditions does a tube vacuum furnace provide for zinc sulfide distillation? Optimize Your Zinc Ore Processing
- What role does an industrial-grade high-temperature tube furnace play in the two-step pyrolysis of Fe–Mn–N–C? Optimize Synthesis
- What is the working environment of a vacuum tube furnace? Achieve Purity and Precision in Material Processing
- What is the specific function of a high-temperature tube furnace for MXene-NiCo2Se4? Master the Selenization Process



















