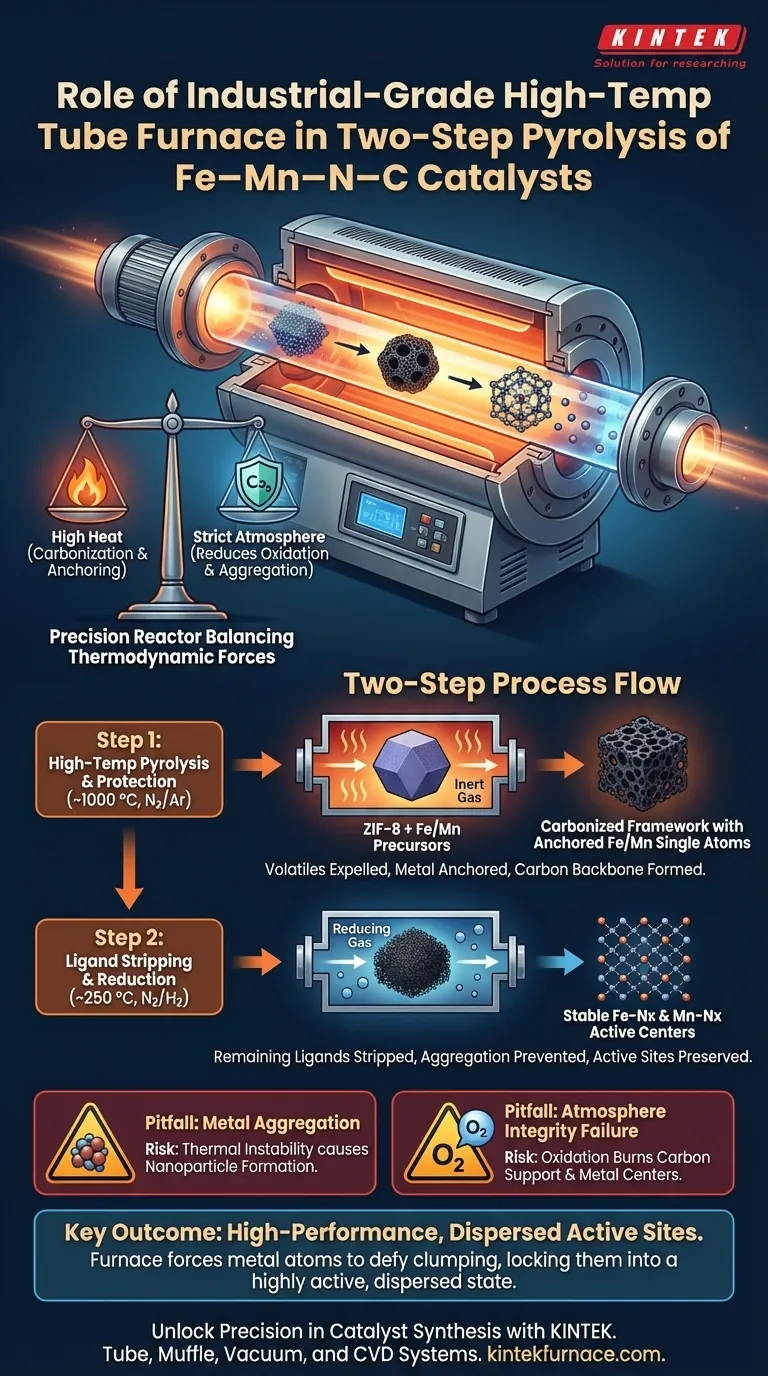
An industrial-grade high-temperature tube furnace serves as the precision reactor required to synthesize high-performance Fe–Mn–N–C catalysts. It provides a tightly sealed, thermally controlled environment that enables the transformation of metal-organic precursors into stable, atomically dispersed active centers. By regulating temperature and atmosphere, the furnace facilitates the carbonization of frameworks like ZIF-8 while preventing the aggregation of iron and manganese atoms.
Core Takeaway The tube furnace’s critical function is to balance thermodynamic forces: it supplies the high heat needed to carbonize the support and anchor metal atoms, yet maintains a strict reducing or inert atmosphere to prevent oxidation and ensure metals remain as isolated, active single or dual atoms.

The Physicochemical Role of the Furnace
Carbonization of the ZIF-8 Framework
At high temperatures, typically around 1000 °C, the tube furnace induces the thermal decomposition of the ZIF-8 precursor. This process converts the organic framework into a conductive, nitrogen-doped carbon substrate. This carbon backbone provides the necessary porosity and surface area to host the catalytic reaction.
Anchoring Active Metal Centers
The thermal energy supplied by the furnace facilitates chemical coordination between the metal sources (Fe and Mn) and nitrogen atoms in the carbon lattice. This effectively "anchors" the metals into the substrate. The result is the formation of high-performance single-atom or dual-atom active centers (Fe-Nx and Mn-Nx), which are far more efficient than bulk metal particles.
Environmental Control in the Two-Step Process
Step 1: High-Temperature Pyrolysis & Protection
During the initial high-temperature stage, the furnace maintains a flow of inert gas (such as Nitrogen or Argon). This protects the precursors from oxidation while expelling volatile decomposition products generated as the organic ligands break down. This creates the porous structure essential for mass transport within the catalyst.
Step 2: Ligand Stripping and Reduction
In the second stage of the two-step process (often around 250 °C), the furnace introduces a reducing atmosphere, such as a mixture of Nitrogen and Hydrogen (N2/H2). This specific environment strips away remaining ligands without overheating the material. Crucially, this prevents the over-oxidation or aggregation of manganese single atoms, preserving the delicate topological structure of the active sites.
Common Pitfalls and Trade-offs
The Risk of Metal Aggregation
The most significant risk in this process is thermal instability. If the temperature spikes uncontrolled or the heating ramp is too aggressive, metal atoms will migrate and clump together to form nanoparticles. The tube furnace’s precision control is the only barrier preventing these high-performance single atoms from degrading into low-activity metallic clusters.
Atmosphere Integrity
A compromised seal or impure gas flow allows oxygen to enter the chamber. At these temperatures, oxygen acts destructively, burning off the carbon support and oxidizing the metal centers. The industrial-grade sealing of the tube furnace is vital to maintain the oxygen-free conditions required for in-situ carbonization and reduction.
Making the Right Choice for Your Goal
When configuring your tube furnace protocols for Fe–Mn–N–C synthesis, align your settings with your specific catalytic targets:
- If your primary focus is Structural Stability: Prioritize precise control at the high-temperature range (1000 °C) to ensure complete graphitization of the ZIF-8 carbon framework.
- If your primary focus is Maximizing Active Site Density: Optimize the second annealing stage (250 °C with N2/H2) to ensure thorough ligand stripping without inducing metal aggregation.
The tube furnace is not just a heater; it is the instrument that forces metal atoms to defy their natural tendency to clump, locking them instead into a highly active, dispersed state.
Summary Table:
| Process Stage | Typical Temp | Atmosphere | Primary Function |
|---|---|---|---|
| Stage 1: Pyrolysis | ~1000 °C | Inert (N2/Ar) | ZIF-8 carbonization & metal anchoring |
| Stage 2: Reduction | ~250 °C | Reducing (N2/H2) | Ligand stripping & preventing atom aggregation |
| Key Outcome | N/A | High Purity | Formation of stable Fe-Nx/Mn-Nx active sites |
Unlock Precision in Catalyst Synthesis with KINTEK
Maximize the activity of your Fe–Mn–N–C catalysts with our industrial-grade heating solutions. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Vacuum, and CVD systems designed to maintain the strict atmospheric integrity and thermal precision required for single-atom dispersion. Whether you need to refine the ZIF-8 carbonization or stabilize delicate dual-atom centers, our customizable furnaces ensure your research translates into scalable results.
Ready to elevate your material performance? Contact us today to find your perfect furnace solution!
Visual Guide
References
- Shiyang Liu, Chuan Zhao. Dual Metal Fe–Mn–N–C Sites with Improved Stability for the Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell. DOI: 10.1002/smtd.202500116
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is a tube furnace with 5% hydrogen/argon mixed atmosphere necessary for PtPd_CoNiCu/C high-entropy alloys?
- How does the controlled atmosphere within a high-temperature tube furnace protect Al-Cr-Cu-Fe-Mn-Ni alloys? Key Insights
- What role does a tube furnace play in converting nickel precursors? Master Thermal Reduction in Argon Atmospheres
- How does a high vacuum tube furnace contribute to the carbonization process? Engineered Hard Carbon Synthesis
- What critical conditions does a tube furnace provide for ZIF-67 pyrolysis? Master Metal/Carbon Nanocomposite Production
- Why are sealed quartz tubes required for Au-Seeded TiO2 nanowires? Ensure Vapor-Phase Stability and VLS Growth
- What makes tube furnaces indispensable in academic and industrial settings? Unlock Precision Heating for Advanced Materials
- What are some common applications of tube furnaces in laboratories? Unlock Precision in Material Processing



















