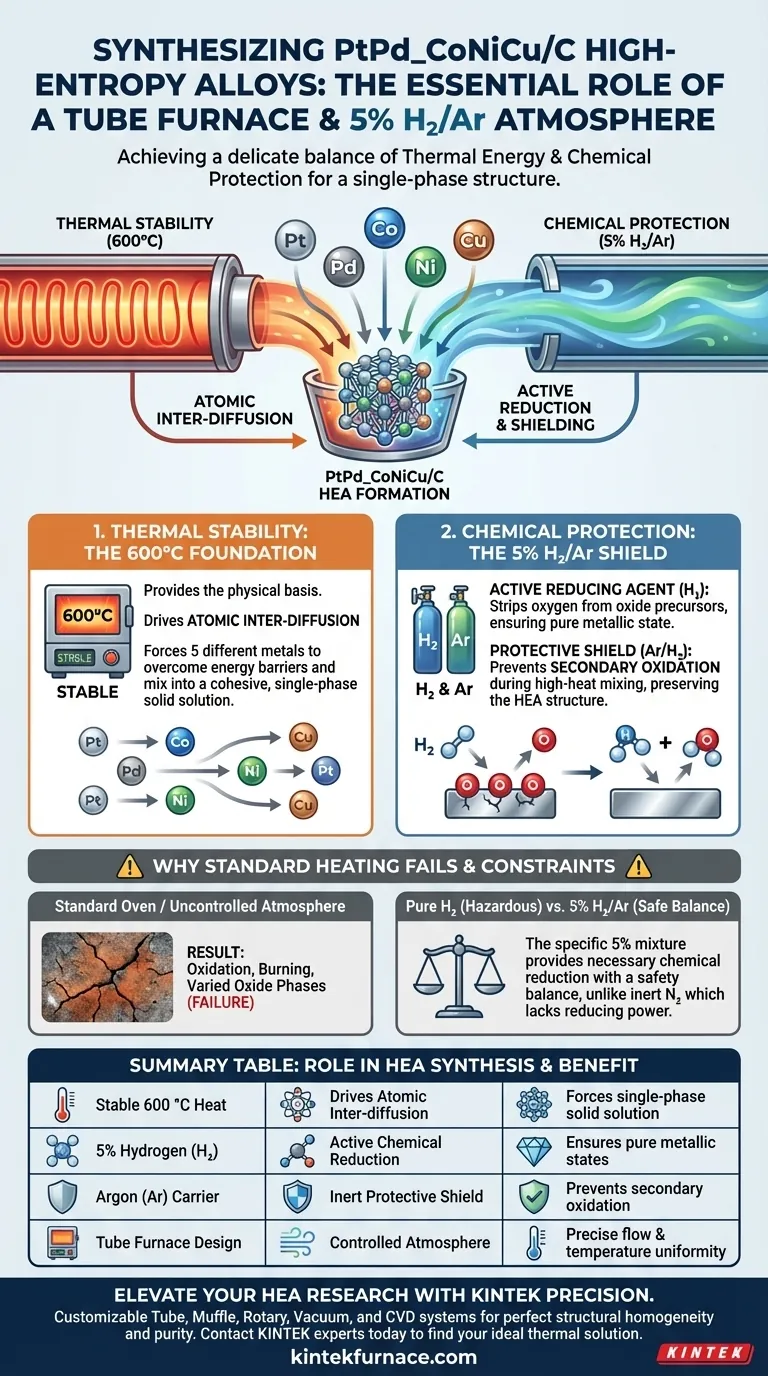
The synthesis of PtPd_CoNiCu/C high-entropy alloys relies on a delicate balance of thermal energy and chemical protection. A tube furnace is necessary to provide a stable 600 °C environment that drives atomic inter-diffusion, while the 5% hydrogen/argon atmosphere is required to chemically strip away oxygen and prevent the metals from oxidizing during this high-heat process.
Core Takeaway To create a high-entropy alloy (HEA), you must force different atoms to mix into a single structure without allowing them to degrade. The tube furnace system provides the thermal energy required for mixing while simultaneously using hydrogen to actively remove impurities and lock in the metallic state.

The Role of Thermal Stability
Establishing the Physical Basis
The primary function of the tube furnace is to maintain a stable 600 °C environment.
This specific thermal condition acts as the physical foundation for the entire synthesis. Without precise temperature control, the reaction kinetics would be unpredictable.
Promoting Atomic Inter-diffusion
For a high-entropy alloy to form, five different metal atoms (Pt, Pd, Co, Ni, Cu) must occupy a single lattice structure.
The 600 °C heat provides the necessary energy for these atoms to overcome energy barriers. This promotes inter-diffusion, allowing the atoms to migrate and mix thoroughly to form a cohesive, single-phase solid solution.
The Function of the Hydrogen Atmosphere
Active Reduction of Precursors
The 5% hydrogen component in the gas mixture serves as an active reducing agent.
Precursors often contain metal oxides or hydroxides rather than pure metal. The hydrogen reacts with these compounds, completely reducing them into a pure metallic state.
Preventing Secondary Oxidation
High temperatures naturally accelerate oxidation, which destroys the integrity of an alloy.
The hydrogen/argon mixture acts as a protective shield. It prevents secondary oxidation from occurring during the 600 °C heating phase, ensuring the final product maintains the required high-entropy alloy (HEA) structure.
Understanding the Constraints and Requirements
Why Standard Heating is Insufficient
Using a standard oven or an uncontrolled atmosphere would result in failure.
Without the reducing atmosphere, the high temperatures required for inter-diffusion would simply burn the metals or form varied oxide phases rather than a unified alloy.
The Importance of the Gas Mixture
Pure hydrogen can be hazardous; a 5% mixture in an inert carrier like Argon provides a safety balance.
While supplementary processes (like biomass carbonization) may rely on Nitrogen, this specific HEA synthesis requires hydrogen because the goal is the chemical reduction of metal species, not just inert protection.
Making the Right Choice for Your Goal
When configuring your synthesis equipment, consider your specific objectives:
- If your primary focus is Structural Homogeneity: Ensure your tube furnace can hold 600 °C with minimal fluctuation to maximize atomic inter-diffusion.
- If your primary focus is Chemical Purity: Verify the flow rate and concentration of the 5% hydrogen mixture to ensure the complete reduction of all oxide precursors.
Success in synthesizing this HEA depends on using heat to drive the mixture and hydrogen to preserve the metal.
Summary Table:
| Requirement | Role in HEA Synthesis | Benefit to PtPd_CoNiCu/C |
|---|---|---|
| Stable 600 °C Heat | Drives Atomic Inter-diffusion | Forces 5 metals into a single-phase solid solution |
| 5% Hydrogen (H2) | Active Chemical Reduction | Strips oxygen from precursors to ensure pure metallic states |
| Argon (Ar) Carrier | Inert Protective Shield | Prevents secondary oxidation during high-heat mixing |
| Tube Furnace Design | Controlled Atmosphere Environment | Maintains precise gas flow and temperature uniformity |
Elevate Your HEA Research with KINTEK Precision
Precision is non-negotiable when synthesizing complex high-entropy alloys like PtPd_CoNiCu/C. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems designed to handle the rigorous demands of chemical reduction and thermal stability.
Our lab high-temperature furnaces are fully customizable to meet your unique atmosphere and temperature profile requirements, ensuring your materials achieve perfect structural homogeneity and purity every time.
Ready to optimize your synthesis process? Contact KINTEK experts today to find the ideal thermal solution for your lab.
Visual Guide
References
- A. K. Nevelskaya, Ilya Pankov. High-Temperature Synthesis of High-Entropy Alloy PtPd_CoNiCu Nanoparticles as a Catalyst for the Oxygen Reduction Reaction. DOI: 10.3390/ijms262311504
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- Why are a high-temperature tube furnace and its reduction atmosphere control system core equipment for SrVO3?
- What types of materials can be processed in a rotary tube furnace? Discover Ideal Materials for High-Temp Processing
- What role does a tube furnace play in the high-temperature modification of La-EPS-C-450? Key Synthesis Insights
- How can the performance of a vertical tube furnace be optimized? Boost Efficiency and Precision in Heat Treatment
- What is the significance of using a tubular furnace in waste salt pyrolysis research? Precision for High-Fidelity Data
- Why is a sealed vacuum quartz tube required for synthesis of 1T-SnS2 via CVT? Ensure Pure Crystal Growth
- How is solid-gas phase conversion achieved in a tube furnace? Master Fe-CoP/CW Catalyst Phosphatization
- How to clean a tube furnace? A Step-by-Step Guide to Safe and Effective Maintenance



















