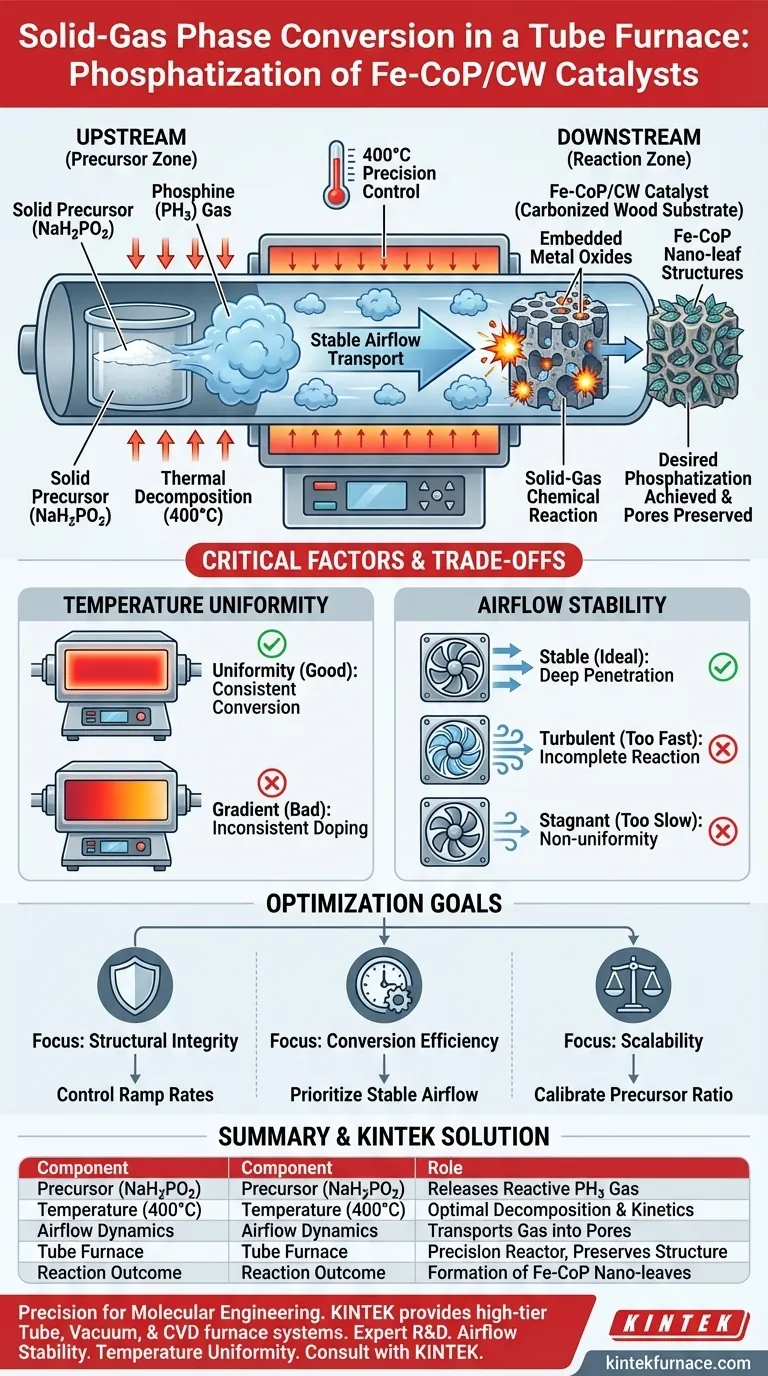
Solid-gas phase conversion is achieved by utilizing the tube furnace to thermally decompose a solid precursor, sodium hypophosphite (NaH2PO2), placed upstream of the catalyst sample. At a controlled temperature of 400°C, this solid releases phosphine (PH3) gas, which is carried by a stable airflow to react directly with the metal oxides embedded within the downstream carbonized wood.
The tube furnace functions not just as a heater, but as a precision flow reactor. It generates the necessary reducing atmosphere in situ, allowing reactive gases to penetrate deep into the material's hierarchical pores and transform the chemical structure without collapsing the physical framework.

The Mechanism of In-Situ Conversion
Thermal Decomposition of the Precursor
The process begins upstream with sodium hypophosphite (NaH2PO2).
Instead of introducing a pre-mixed gas from an external tank, the furnace uses thermal energy to break down this solid salt.
This decomposition releases phosphine (PH3), a highly reactive reducing gas, which serves as the phosphorus source for the conversion.
Gas Transport and Penetration
Once generated, the PH3 gas does not remain static.
A stable, directed airflow transports the gas downstream toward the Fe-CoP/CW catalyst precursor.
Because the gas is generated within the flow path, it can effectively penetrate the hierarchical pores of the carbonized wood substrate.
Chemical Transformation
The core reaction happens at the site of the metal oxides.
The PH3 gas engages in a thorough solid-gas chemical reaction with the loaded metal oxides.
This transforms the nanosheets into highly dispersed Fe-CoP nano-leaf structures, achieving the desired phosphatization.
The Role of the Thermal Environment
Precision Temperature Control
Success depends on maintaining a specific thermal energy level.
The primary reference indicates that a constant temperature of 400°C is required for this specific conversion.
This temperature is sufficient to decompose the precursor and drive the reaction kinetics but prevents thermal degradation of the carbonized wood.
Preserving Pore Architecture
The tube furnace environment protects the structural integrity of the catalyst.
Unlike wet chemical methods which might collapse fragile structures, this gas-phase treatment preserves the "hierarchical pores" of the wood.
This ensures that the final catalyst retains a high surface area for active sites.
Understanding the Trade-offs
Airflow Stability
The "stable airflow" mentioned in the primary reference is a critical variable, not just a feature.
If the airflow is too turbulent, the PH3 gas may pass over the sample too quickly, leading to incomplete phosphatization.
If the airflow is too stagnant, the gas may not penetrate the deeper pores, resulting in non-uniform surface chemistry.
Temperature Uniformity
While the target is 400°C, the gradient within the tube matters.
The furnace must ensure that both the upstream precursor (for decomposition) and the downstream sample (for reaction) are within their required thermal windows.
A failure in constant temperature control can lead to inconsistent doping or partial conversion of the metal oxides.
Making the Right Choice for Your Goal
To optimize the phosphatization of Fe-CoP/CW catalysts, consider your specific processing objectives:
- If your primary focus is Structural Integrity: Ensure the temperature ramp rates are controlled to prevent thermal shock to the carbonized wood skeleton.
- If your primary focus is Chemical Conversion Efficiency: Prioritize the stability of the airflow to ensure maximum residence time of the PH3 gas within the hierarchical pores.
- If your primary focus is Scalability: Calibrate the ratio of upstream NaH2PO2 to the downstream sample mass to ensure a sufficient surplus of PH3 gas for larger batches.
Mastering the airflow and temperature precision turns a simple tube furnace into a sophisticated tool for molecular engineering.
Summary Table:
| Process Component | Role in Phosphatization |
|---|---|
| Precursor (NaH2PO2) | Thermally decomposes to release reactive PH3 gas |
| Process Temperature | Fixed at 400°C for optimal decomposition and kinetics |
| Airflow Dynamics | Transports gas downstream into hierarchical pores |
| Tube Furnace Environment | Precision flow reactor preserving structural integrity |
| Reaction Outcome | Transformation of metal oxides into Fe-CoP nano-leaf structures |
Precision is the difference between a failed reaction and a high-performance catalyst. KINTEK provides high-tier Tube, Vacuum, and CVD furnace systems engineered for rigorous solid-gas phase conversions. Backed by expert R&D and manufacturing, our systems ensure the airflow stability and temperature uniformity required to preserve delicate hierarchical architectures in materials like carbonized wood. Consult with KINTEK today to customize a high-temperature solution for your unique molecular engineering needs.
Visual Guide
References
- Yuan Ma, Jie Gao. Boosting electrocatalytic generation of FDCA and H2 from 2,5-furanedimethanol solution by carbonized wood supported Fe-CoP nanoleaves. DOI: 10.1007/s42773-024-00380-9
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is a fixed-bed tubular reactor used for the in-situ reduction process? Enhance Catalyst Activation Efficiency
- What is a tube furnace and what are its primary uses? Essential for Controlled High-Temperature Processes
- What is the specific role of a tube furnace in the pre-treatment of activated carbon catalysts? Precision Modification
- Why is a tube furnace with flowing nitrogen required for Cu/Zn-SAN pyrolysis? Achieve Atomic Dispersion
- What is the difference between a tube furnace and a muffle furnace? Choose the Right High-Temp Solution
- What are the advantages of decomposing tube furnaces? Achieve Precise Control and High Efficiency in Thermal Processes
- How does a high-temperature tube furnace ensure magnesium alloy scaffold performance? Expert Sintering Guide
- What is the primary function of a high-temperature tube furnace in NaF–Na3AlF6 molten salt experiments? Learn more!



















