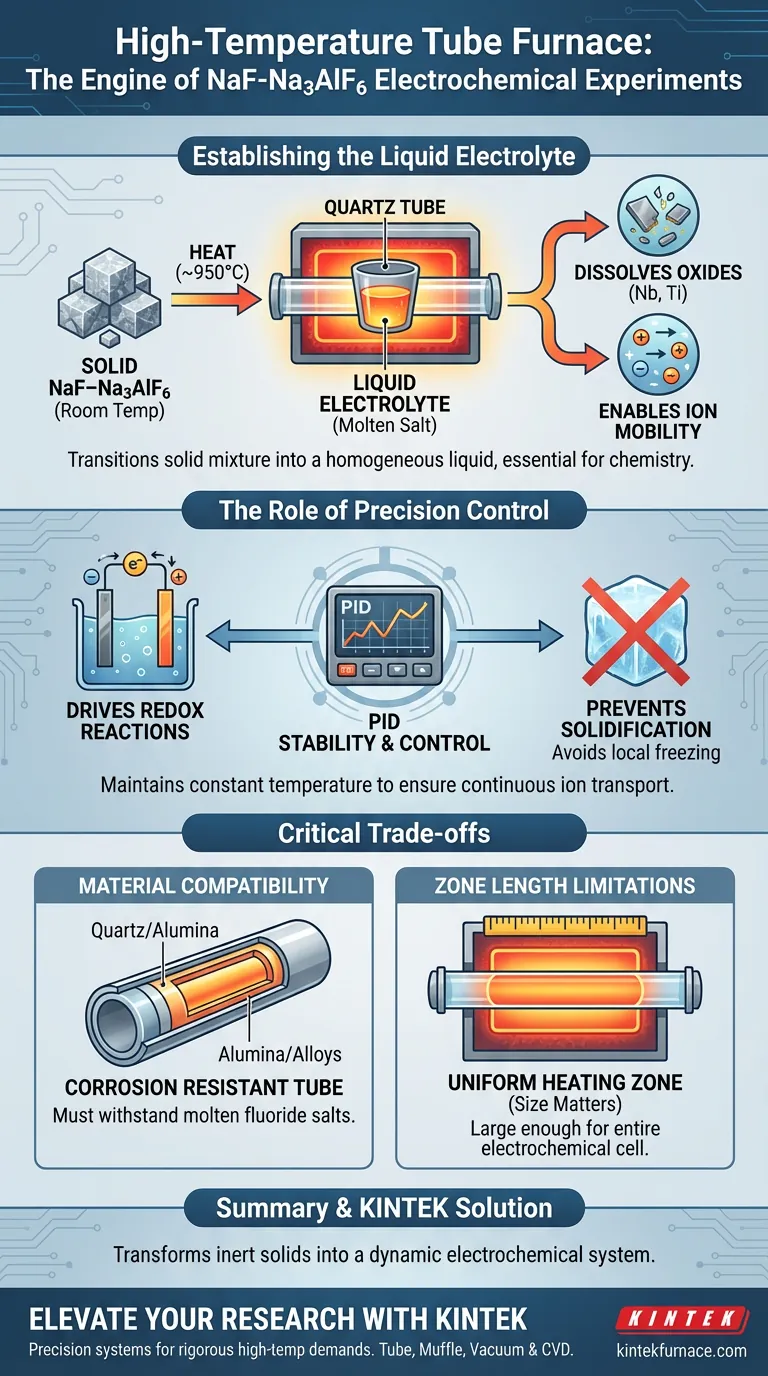
The primary function of a high-temperature tube furnace in NaF–Na3AlF6 experiments is to provide a precise, stable thermal environment, typically maintained at 950°C. This specific heat level is required to completely transition the solid sodium fluoride-cryolite mixture into a homogeneous liquid electrolyte. Without this phase change, the electrochemical system cannot function.
Core Takeaway The tube furnace does not merely heat the sample; it creates the fundamental liquid phase environment necessary for chemistry to occur. By maintaining a constant 950°C, it ensures the dissolution of metal oxides and enables the ion migration required for redox reactions.

Establishing the Liquid Electrolyte
Achieving the Melting Point
The NaF–Na3AlF6 system is solid at room temperature and electrically insulating. The furnace must reach high temperatures (often 950°C) to fully melt this cryolite-based system.
Creating a Solvent for Oxides
Once liquefied, the molten salt creates a medium capable of dissolving specific metal oxides. In these specific experiments, this environment allows for the dissolution of niobium and titanium oxides.
Enabling Ion Mobility
Electrochemical reactions rely on the movement of ions between electrodes. The furnace converts the static solid lattice into a fluid state, facilitating the ion migration necessary to complete the electrical circuit.
The Role of Precision Control
Driving Redox Reactions
The ultimate goal of the experiment is the electrochemical reduction (redox reaction) of the dissolved oxides. The thermal energy provided by the furnace lowers the activation energy required for these reactions to proceed efficiently.
Maintaining Thermal Stability
The tube furnace typically employs advanced PID controllers to ensure the temperature remains stable rather than fluctuating.
Preventing Localized Solidification
Stability is critical because any significant drop in temperature can cause the salt to solidify locally. This would immediately halt ion transport and disrupt the electrolysis process.
Understanding the Trade-offs
Material Compatibility
While tube furnaces provide excellent heat control, the reaction tube material is a critical constraint. Whether using quartz, alumina, or metal alloys, the tube must withstand the corrosive nature of molten fluoride salts at 950°C without contaminating the electrolyte.
Zone Length Limitations
The heating zone length (typically 205 mm to 1200 mm) dictates the size of your electrochemical cell. You must ensure the constant temperature zone is large enough to encompass the entire cell to avoid temperature gradients, which can alter electrolyte viscosity and conductivity.
Making the Right Choice for Your Goal
The selection and operation of your furnace should be dictated by your specific experimental parameters.
- If your primary focus is reaction stability: Prioritize a furnace with high-precision PID control to maintain constant viscosity and conductivity within the electrolyte.
- If your primary focus is scaling up the cell: Ensure the furnace's heating zone length is sufficient to provide a uniform thermal field across the entire larger apparatus.
A high-temperature tube furnace is the foundational tool that transforms inert solids into a dynamic electrochemical system.
Summary Table:
| Feature | Function in NaF–Na3AlF6 Experiments |
|---|---|
| Temperature Target | Typically 950°C to ensure complete melting |
| Phase Transition | Converts solid cryolite mixture into a liquid electrolyte |
| Ion Mobility | Facilitates ion migration required for redox reactions |
| Thermal Stability | PID control prevents solidification and ensures conductivity |
| Solvent Action | Enables the dissolution of niobium and titanium oxides |
Elevate Your Electrochemical Research with KINTEK
Precision is non-negotiable in molten salt electrolysis. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Tube, Muffle, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of high-temperature lab environments. Whether you are working with corrosive fluoride salts or complex oxide reductions, our customizable furnaces provide the thermal stability and uniform heating zones your research requires.
Ready to optimize your high-temp experiments? Contact us today to discuss your unique needs with our technical specialists and discover how KINTEK can empower your laboratory's success.
Visual Guide
References
- Bo Zhang, Maofa Jiang. Electrochemical Behavior of Niobium Oxide and Titanium Oxide in NaF–Na3AlF6 Molten Salt. DOI: 10.3390/met14030297
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What factors influence the processing time in a rotary tube furnace? Master Control for Efficient Heat Treatment
- What are the primary functions of a tube furnace in the thermal pre-treatment research of aluminum alloy powder?
- What is the advantage of a three-zone tube furnace? Achieve Larger, More Uniform Heating for Your Processes
- What are the key design features of a split tube furnace? Unlock Superior Access for Complex Experiments
- What is the function of a Tube Furnace during molybdenum carbide synthesis? Master Catalyst Carbonization
- What role does an Electrically Heated Drop Tube Furnace (DTF) play in iron powder experiments? Boost Your Research Now!
- Why is a tube annealing furnace used for SiC hydrogenation? Unlock Pure Atomic Surfaces for Superior Crystal Bonding
- What is the function of a two-zone tube furnace in Borophene CVD? Achieve Precise Thermal Decoupling for 2D Synthesis



















