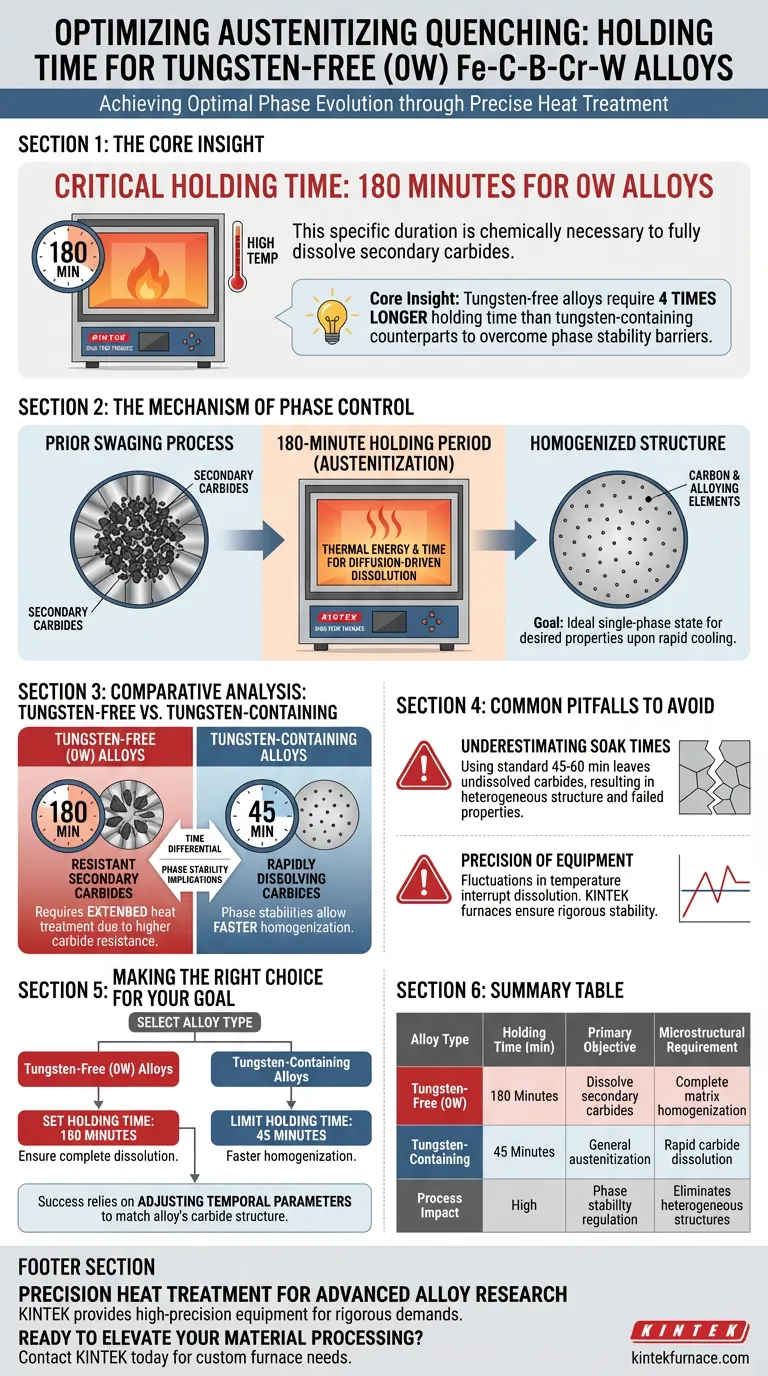
To ensure optimal phase evolution, the holding time in a high-temperature laboratory furnace for tungsten-free (0W) Fe-C-B-Cr-W alloys must be maintained at 180 minutes. This specific duration is chemically necessary to fully dissolve the large quantity of secondary carbides generated during prior swaging processes back into the matrix.
Core Insight: While standard austenitization seeks general homogenization, tungsten-free alloys require a significantly extended holding time—four times longer than their tungsten-containing counterparts—to overcome the specific phase stability barriers created by secondary carbides.
The Mechanism of Phase Control
Dissolving Secondary Carbides
The swaging process creates a dense population of secondary carbides within the alloy structure.
For the material to achieve the correct properties during quenching, these carbides must be completely dissolved back into the matrix.
The 180-minute holding period provides the necessary thermal energy and time for this diffusion-driven dissolution to occur effectively.
Achieving Homogenization
The ultimate goal of this holding period is to achieve a fully homogenized structure.
By maintaining the temperature for the prescribed time, the furnace ensures the carbon and alloying elements are evenly distributed.
This creates an ideal single-phase state, which is the prerequisite for obtaining the desired microstructure upon rapid cooling.
Comparative Analysis: Tungsten-Free vs. Tungsten-Containing
The Time Differential
There is a distinct disparity in processing requirements based on chemical composition.
Tungsten-containing alloys require a holding time of only 45 minutes.
In stark contrast, tungsten-free (0W) alloys demand 180 minutes to achieve the same level of microstructural readiness.
Phase Stability Implications
This difference highlights how the removal of tungsten alters the thermodynamic stability of the phases.
The tungsten-free composition results in carbides that are more resistant to dissolution or simply present in greater quantities that require longer soak times.
Therefore, laboratory protocols cannot be standardized across these alloy types; the lack of tungsten necessitates a specifically tailored, extended heat treatment.
Common Pitfalls to Avoid
Underestimating Soak Times
The most critical error in processing 0W alloys is applying standard holding times (e.g., 45 to 60 minutes) used for other alloy variants.
Insufficient holding time will leave undissolved secondary carbides in the matrix.
This results in a heterogeneous structure that will fail to develop the intended material properties after quenching.
Precision of Equipment
The laboratory furnace must be capable of rigorous stability over long durations.
Fluctuations in temperature during the extended 180-minute cycle can interrupt the dissolution process or lead to uneven phase regulation.
Precise control of the furnace parameters is the only way to regulate the microscopic phase components accurately.
Making the Right Choice for Your Goal
To ensure you achieve the correct microstructural baseline for your specific alloy, apply the following protocols:
- If your primary focus is Tungsten-Free (0W) Alloys: Set your furnace holding time to exactly 180 minutes to ensure complete dissolution of swaging-induced secondary carbides.
- If your primary focus is Tungsten-Containing Alloys: Limit your holding time to 45 minutes, as the phase stabilities in this composition allow for much faster homogenization.
Success in this process relies entirely on adjusting your temporal parameters to match the specific dissolution requirements of the alloy's carbide structure.
Summary Table:
| Alloy Type | Holding Time (min) | Primary Objective | Microstructural Requirement |
|---|---|---|---|
| Tungsten-Free (0W) | 180 Minutes | Dissolve secondary carbides | Complete matrix homogenization |
| Tungsten-Containing | 45 Minutes | General austenitization | Rapid carbide dissolution |
| Process Impact | High | Phase stability regulation | Eliminates heterogeneous structures |
Precision Heat Treatment for Advanced Alloy Research
Achieving the perfect phase dissolution in tungsten-free alloys requires unwavering thermal stability over extended 180-minute cycles. KINTEK provides the high-precision equipment necessary for such rigorous laboratory demands.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Our lab high-temp furnaces are fully customizable to meet the unique heating profiles of your specific materials, ensuring consistent, reproducible results every time.
Ready to elevate your material processing? Contact KINTEK today to discuss your custom furnace needs and see how our expertise can streamline your research and production.
Visual Guide
References
- H. SCHAEFER, Sebastian Weber. Microstructure Formation in Hypoeutectic Alloys in the Fe–C–B–Cr–W System. DOI: 10.1007/s11661-024-07675-3
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is a vacuum oven required during the preparation of Al-CNTs/h-BN composites? Prevent Defects & Ensure Purity
- What is the purpose of treating ADSC powders with hydrogen? Purify Your Material for Superior Conductivity
- What is a horizontal furnace? A space-saving heating solution for attics and crawl spaces
- How does a vacuum drying oven provide superior performance for MoS2/C powders? Preserve Purity and Nanostructure
- What is the function of a sintering aid reservoir? Unlock Rapid Densification via MV-Sintering Technology
- What is the importance of a laboratory oven's programmed heating for epoxy-polyimide curing? Essential Thermal Control
- How does a crucible furnace work? A Guide to Efficient Metal Melting
- What is the significance of using a 650°C annealing furnace for 42CrMo/Cr5 composite rollers after forging?



















