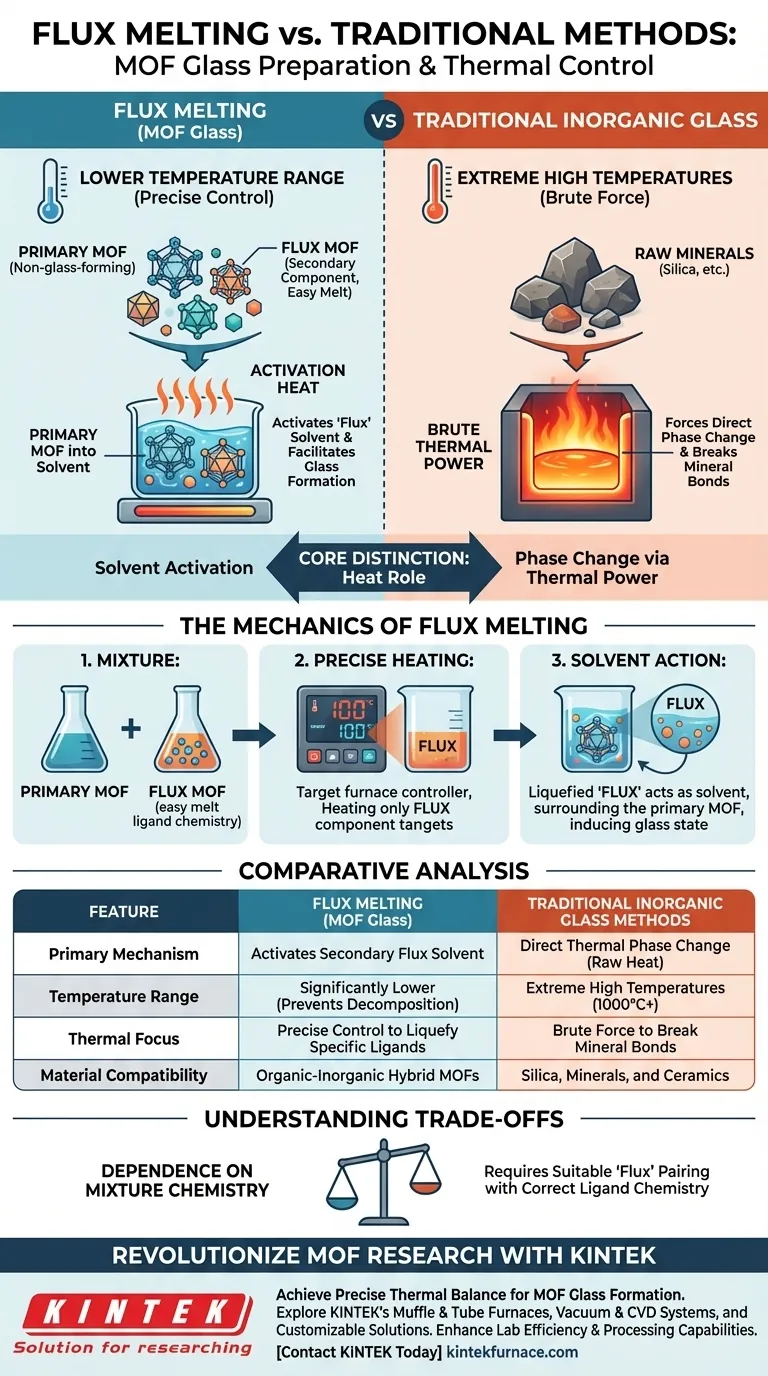
In the flux melting process, heating equipment is utilized to target a specific, lower temperature range that liquefies a secondary "flux" component rather than melting the entire bulk material at high heat. Unlike traditional inorganic glass methods that rely on extreme temperatures to force a phase change, this technique uses precise thermal control to activate a secondary Metal-Organic Framework (MOF) as a solvent, facilitating glass formation at significantly reduced temperatures.
The core distinction lies in utilizing heat to activate a solvent rather than to force a phase change through raw thermal power. By melting a sacrificial "flux" MOF, the equipment creates a liquid environment that allows non-glass-forming materials to vitrify without reaching their decomposition temperatures.

The Mechanics of the Flux Melting Process
The Role of the "Flux" Component
The process begins by mixing a non-glass-forming MOF with a second, specific MOF. This second component is selected because its ligand chemistry allows it to melt easily.
Thermal Precision Over Brute Force
The heating equipment is not set to the high melting point of the primary material. Instead, it is set to precisely control the temperature to liquefy only the easily melting component.
Creating a Liquid Solvent
Once the second MOF melts, it acts as a "solvent" or "flux." This liquid phase surrounds the non-glass-forming MOF, inducing the entire mixed system to transition into a glass state.
Comparative Analysis: MOF Flux vs. Traditional Methods
Temperature Requirements
Traditional inorganic glass preparation generally requires heating equipment capable of reaching extreme temperatures to melt raw silica or other minerals directly.
In contrast, the flux melting process allows for glass formation at lower temperatures. This is critical for MOFs, which are organic-inorganic hybrids that might decompose under the intense heat required for traditional methods.
The Mechanism of Action
Traditional methods rely on heat alone to break bonds and create a liquid.
The flux melting method functions similarly to molten salt methods. The heating equipment facilitates a chemical environment where the flux does the work of liquefying the system, effectively expanding the range of materials that can be processed.
Understanding the Trade-offs
Dependence on Mixture Chemistry
This process is not universal for all single-component materials. It relies heavily on the compatibility of the mixture.
You must successfully pair a non-glass-forming MOF with a suitable "flux" MOF that has the correct ligand chemistry to melt easily without degrading the primary structure.
Making the Right Choice for Your Goal
To determine if flux melting is the appropriate processing route for your material, consider your primary constraints:
- If your primary focus is processing non-glass-forming MOFs: The flux melting method is essential, as it induces glass formation in materials that cannot form glass on their own.
- If your primary focus is thermal stability: This method allows you to process materials at lower temperatures, avoiding the thermal decomposition associated with traditional high-heat methods.
By using the flux component as a solvent, you bypass the thermal limits of traditional glass manufacturing.
Summary Table:
| Feature | Flux Melting (MOF Glass) | Traditional Inorganic Glass Methods |
|---|---|---|
| Primary Mechanism | Activation of a secondary "flux" solvent | Direct thermal phase change (raw heat) |
| Temperature Range | Significantly lower (prevents decomposition) | Extreme high temperatures (1000°C+) |
| Thermal Focus | Precise control to liquefy specific ligands | Brute force to break mineral bonds |
| Material Compatibility | Organic-inorganic hybrid MOFs | Silica, minerals, and ceramics |
| Role of Heat | Facilitates a chemical liquid environment | Physically melts the bulk material |
Revolutionize Your MOF Research with Precision Thermal Solutions
Are you looking to master the delicate thermal balance required for MOF glass formation? KINTEK provides the cutting-edge heating equipment necessary to achieve the precise temperature control that flux melting demands.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab high-temp systems, including:
- Muffle & Tube Furnaces: For uniform heating and precise ligand melting.
- Vacuum & CVD Systems: Ideal for sensitive organic-inorganic hybrid processing.
- Customizable Solutions: Tailored to the unique chemistry of your specific flux and MOF pairings.
Don't risk material decomposition with imprecise equipment. Contact KINTEK today to discuss how our specialized furnaces can enhance your lab's efficiency and expand your material processing capabilities.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the purpose of using a vacuum drying oven for coated carbon cloth? Protect Substrates & Optimize Adhesion
- How does Electroslag Remelting (ESR) technology enhance Ni30 superalloys? Unlock Maximum Purity and Plasticity
- Why is temperature control precision critical for gear steel pseudo-carburizing? Ensure Valid Microstructural Results
- How do chill rings specifically influence the temperature field distribution? Expert Insight into Crystal Casting
- What is the function of a vacuum drying oven in SFRP processing? Preserve Material Integrity & Prevent Degradation
- What is the importance of controlling gas flow rates during purging? Prevent Thermal Stress and Equipment Failure
- What is the importance of the feeding system and ore distributing device? Unlock Peak Oil Shale Retorting Efficiency
- What is the role of a high-temperature stainless steel autoclave in the synthesis of Copper Ferrite (CuFe2O4)?



















