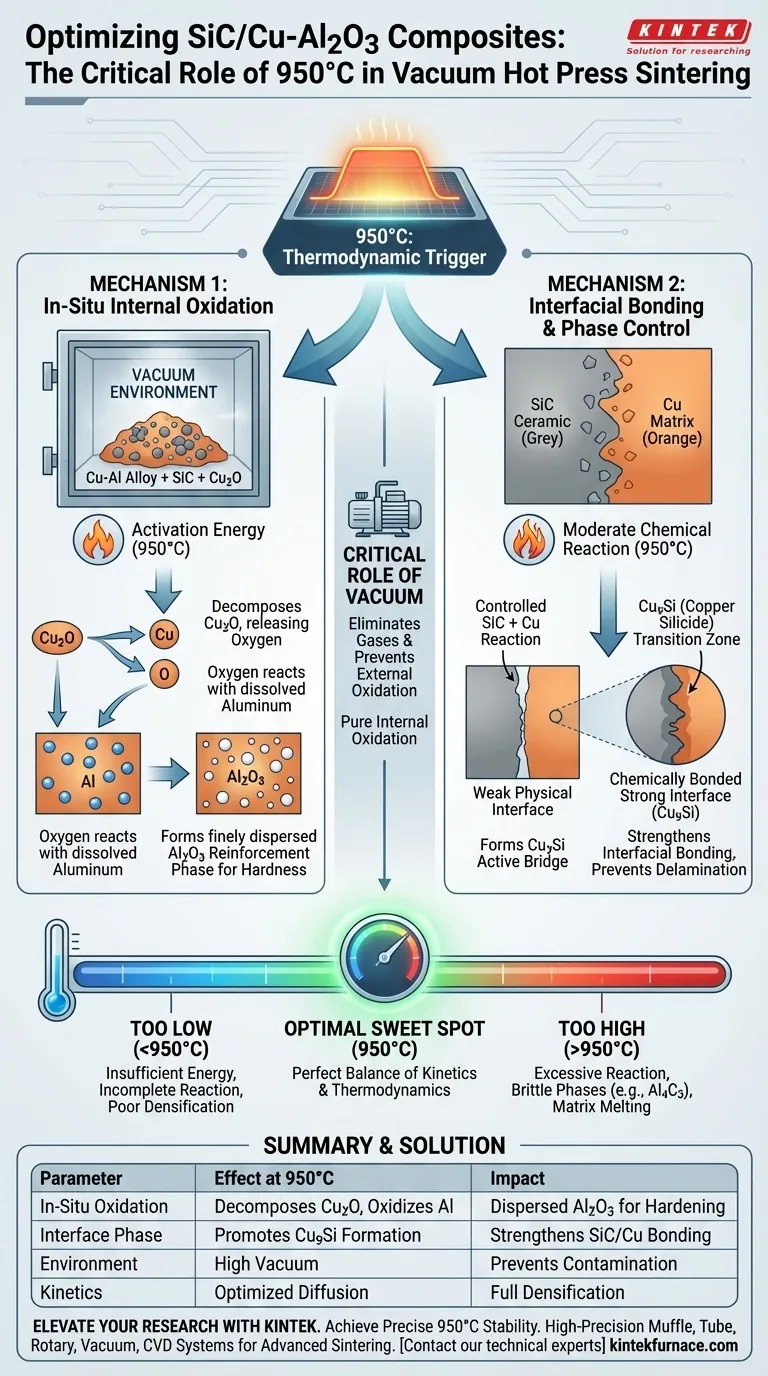
Precise temperature control at 950°C serves as the thermodynamic trigger that dictates the microstructure of SiC/Cu-Al2O3 composites. At this specific thermal plateau, the system acts as a controlled reactor, converting internal chemical potential into mechanical strength by enabling the in-situ internal oxidation of aluminum and optimizing interfacial bonding.
Core Takeaway Regulating the sintering process at 950°C provides the exact activation energy required to decompose Cu2O and oxidize aluminum within the matrix, creating a dispersed Al2O3 reinforcement phase. Simultaneously, this temperature fosters a controlled reaction between SiC and Copper to form Cu9Si, transforming a potentially weak physical interface into a chemically bonded, high-strength transition zone.

The Mechanism of In-Situ Internal Oxidation
Providing Essential Activation Energy
The internal oxidation reaction is not spontaneous at room temperature; it requires a specific energy threshold to initiate.
By holding the temperature at 950°C, you provide the necessary activation energy to destabilize the Cuprous Oxide (Cu2O) present in the raw materials.
This thermal input allows the oxygen atoms to dissociate from the copper and diffuse toward the aluminum dissolved in the Cu-Al alloy.
Formation of the Reinforcing Phase
Once the oxygen is released, the high affinity of aluminum for oxygen drives the formation of Aluminum Oxide (Al2O3).
Because this happens in-situ (within the material during processing) rather than by adding external ceramic powder, the resulting Al2O3 particles are finely dispersed throughout the matrix.
This dispersion is critical for obstructing dislocation motion, which directly enhances the hardness and strength of the composite.
The Critical Role of the Vacuum
While temperature drives the reaction, the vacuum environment ensures the reaction's purity.
The vacuum eliminates interstitial gases and prevents external air from oxidizing the copper matrix.
This ensures that the oxidation of aluminum is strictly internal, controlled solely by the decomposition of Cu2O rather than uncontrolled atmospheric contamination.
Interfacial Bonding and Phase Control
Strengthening the SiC/Cu Interface
A common failure point in metal-ceramic composites is the interface between the ceramic reinforcement (SiC) and the metal matrix (Cu).
At 950°C, the thermal energy induces a moderate chemical reaction between the Silicon Carbide and the Copper matrix.
The Role of Cu9Si
This reaction generates Copper Silicide (Cu9Si).
Unlike brittle contaminants often found in poorly controlled processes, Cu9Si at this specific condition acts as a chemically active bridge.
It strengthens the interfacial bonding, ensuring effective load transfer between the matrix and the reinforcement, which prevents delamination under stress.
Understanding the Trade-offs
The Consequence of Low Temperatures
If the temperature drops significantly below the 950°C target, the system fails to reach the activation energy threshold.
Without sufficient heat, the diffusion of atoms slows down, and the internal oxidation reaction remains incomplete.
This leads to insufficient densification and a lack of the Al2O3 reinforcing phase, resulting in a material with poor mechanical properties.
The Dangers of Excessive Heat
Exceeding the optimal temperature window introduces severe risks.
While 950°C promotes beneficial Cu9Si formation, significantly higher temperatures can trigger aggressive interfacial reactions.
This creates excessive brittle phases (such as Al4C3 in aluminum-rich regions) or leads to matrix melting, which degrades ductility and makes the composite prone to catastrophic fracture.
Making the Right Choice for Your Goal
To optimize the performance of your SiC/Cu-Al2O3 composites, you must view temperature not just as a setting, but as a reactant.
- If your primary focus is Maximum Hardness: Ensure the temperature dwell time at 950°C is sufficient to fully complete the Cu2O decomposition, maximizing the volume fraction of dispersed Al2O3.
- If your primary focus is Interfacial Integrity: Monitor the temperature stability closely to generate the Cu9Si transition layer without overshooting into the range where brittle carbides form.
Success in this process relies on maintaining the thermal "sweet spot" where diffusion kinetics and reaction thermodynamics perfectly align.
Summary Table:
| Parameter | Effect at 950°C | Impact on Composite Property |
|---|---|---|
| In-Situ Oxidation | Decomposes Cu2O to oxidize Al | Creates dispersed Al2O3 for hardening |
| Interface Phase | Promotes Cu9Si formation | Strengthens SiC/Cu chemical bonding |
| Environment | High Vacuum | Prevents matrix contamination/oxidation |
| Kinetics | Optimized diffusion rate | Ensures full densification & load transfer |
Elevate Your Material Research with KINTEK
Precision is the difference between a brittle failure and a high-performance composite. Backed by expert R&D and manufacturing, KINTEK offers high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the rigorous 950°C stability required for your advanced sintering processes.
Whether you are optimizing SiC/Cu-Al2O3 composites or developing new metal-ceramic alloys, our lab high-temp furnaces provide the thermal accuracy and vacuum integrity your innovation demands.
Contact our technical experts today to discuss your unique needs and discover how KINTEK can enhance your lab's efficiency.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- How does hot pressing improve mechanical properties of materials? Achieve Superior Strength and Durability
- What role does hot pressing play in materials science? Achieve High-Density, Complex Parts Efficiently
- How does automation enhance the hot pressing process? Boost Precision, Efficiency, and Quality
- Why is 'final short-time pressing' important in vacuum hot pressing? Unlock Maximum Material Density
- What are the primary applications of vacuum hot press furnaces? Achieve Superior Material Density and Purity
- How does a vacuum press machine work in shaping metals? Achieve Precision Metal Forming with Uniform Pressure
- Why is high-strength graphite selected for vacuum hot pressing of thermoelectric alloys? High-Heat Stability Expert
- Why is vacuum press technology indispensable in modern metalworking? Unlock Precision and Quality in Metal Forming



















