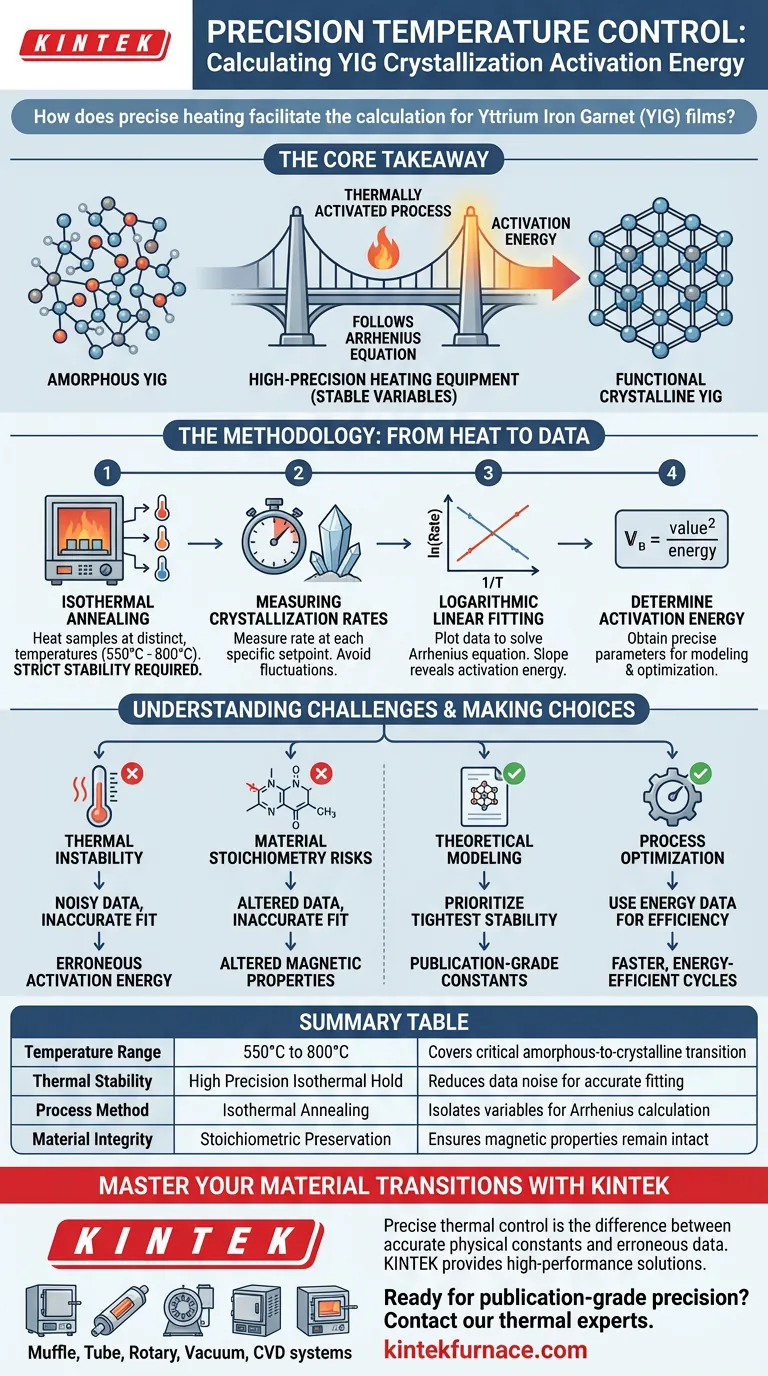
Precise temperature control enables the isolation of crystallization rates at specific, stable intervals, typically between 550°C and 800°C. By maintaining these exact thermal conditions, researchers can generate the consistent data required to perform logarithmic linear fitting, which effectively solves the Arrhenius equation to determine the activation energy of Yttrium Iron Garnet (YIG) films.
The Core Takeaway Crystallization is a thermally activated process that strictly follows the Arrhenius equation. High-precision heating equipment acts as the bridge between theory and practice, allowing you to stabilize variables during isothermal annealing to accurately calculate the energy required to transform YIG films from an amorphous to a functional crystalline state.

The Physics of Thermal Activation
The Transition to Functionality
Yttrium Iron Garnet (YIG) films begin in an amorphous state which lacks the necessary magnetic order for high-performance applications. To become useful for technologies like spintronics, the atomic structure must be reorganized into a crystalline lattice.
The Arrhenius Relationship
This reorganization is not random; it is a thermally activated process. This means the rate at which the film crystallizes is directly dependent on temperature, governed mathematically by the Arrhenius equation.
The Role of Activation Energy
The activation energy is the specific energy barrier the material must overcome to begin crystallizing. determining this parameter is critical because it provides the theoretical basis for optimizing mass production processes.
The Methodology: From Heat to Data
Isothermal Annealing
To calculate activation energy, you cannot simply heat the material once. You must perform isothermal annealing, which involves heating samples at distinct, constant temperatures across a gradient, typically ranging from 550°C to 800°C.
Measuring Crystallization Rates
At each specific temperature setpoint, the high-temperature laboratory system measures how quickly the crystallization occurs. Stability is paramount here; even minor fluctuations in temperature can skew the rate data, rendering the calculation invalid.
Logarithmic Linear Fitting
Once the rates for different temperatures are collected, researchers apply logarithmic linear fitting to the data. By plotting the natural logarithm of the crystallization rate against the inverse of the temperature, the slope of the resulting line reveals the unique activation energy parameters for the YIG system.
Understanding the Challenges
The Cost of Thermal Instability
If the heating equipment cannot maintain a rigorous hold at the target temperature, the observed crystallization rate will not reflect a true isothermal state. This introduces noise into the data, making the linear fit inaccurate and leading to erroneous activation energy calculations.
Material Stoichiometry Risks
While heating provides the energy for crystallization, the environment must also preserve the film's chemical makeup. The furnace must ensure the atomic structure reorganizes without altering the chemical stoichiometry, which is vital for maintaining the film's intended magnetic characteristics.
Making the Right Choice for Your Goal
To effectively utilize crystallization activation energy in your work, consider your primary objective:
- If your primary focus is Theoretical Modeling: Prioritize equipment with the tightest temperature stability to ensure your logarithmic linear fitting yields precise, publication-grade physical constants.
- If your primary focus is Process Optimization: Use the calculated activation energy to design faster, more energy-efficient production cycles that reliably transition films to their crystalline state without overheating.
Precise thermal control transforms raw heat into the quantitative data needed to master YIG film production.
Summary Table:
| Feature | Requirement for YIG Calculation | Impact on Activation Energy Data |
|---|---|---|
| Temperature Range | 550°C to 800°C | Covers critical transition from amorphous to crystalline |
| Thermal Stability | High Precision Isothermal Hold | Reduces data noise for accurate logarithmic linear fitting |
| Process Method | Isothermal Annealing | Isolates variables to solve the Arrhenius equation |
| Material Integrity | Stoichiometric Preservation | Ensures magnetic properties remain intact during heating |
Master Your Material Transitions with KINTEK
Precise thermal control is the difference between accurate physical constants and erroneous data. KINTEK provides the high-performance heating solutions required for sensitive crystallization studies. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet the rigorous stability demands of YIG film research and spintronics applications.
Ready to achieve publication-grade precision? Contact our thermal experts today to find the perfect system for your lab.
Visual Guide
References
- Sebastian Sailler, Michaela Lammel. Crystallization dynamics of amorphous yttrium iron garnet thin films. DOI: 10.1103/physrevmaterials.8.043402
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the differences between solid and split tube furnaces? Choose the Right Furnace for Your Lab
- What role does a tube furnace play in g-C3N4 thin film preparation? Optimize Your Hot-Wall CVD Synthesis
- What structural advantages do vacuum tube furnaces offer? Achieve Purity and Precision in Material Processing
- What are the dimensions and temperature capabilities of single zone horizontal tube furnace models? Explore Key Specs for Your Lab
- What are the current market trends for 70mm tube furnaces? Discover Key Drivers in Automation and High-Tech Applications
- How does a high-temperature tube furnace facilitate the conversion of Cu@ZIF-8? Master Precision Material Synthesis
- Why is vacuum-sealed quartz tube encapsulation necessary? Ensure High-Purity Liquid Metal Spectral Analysis
- What types of reactions can tube furnaces be used for besides synthesis and purification? Explore Versatile Thermal Processing Applications



















