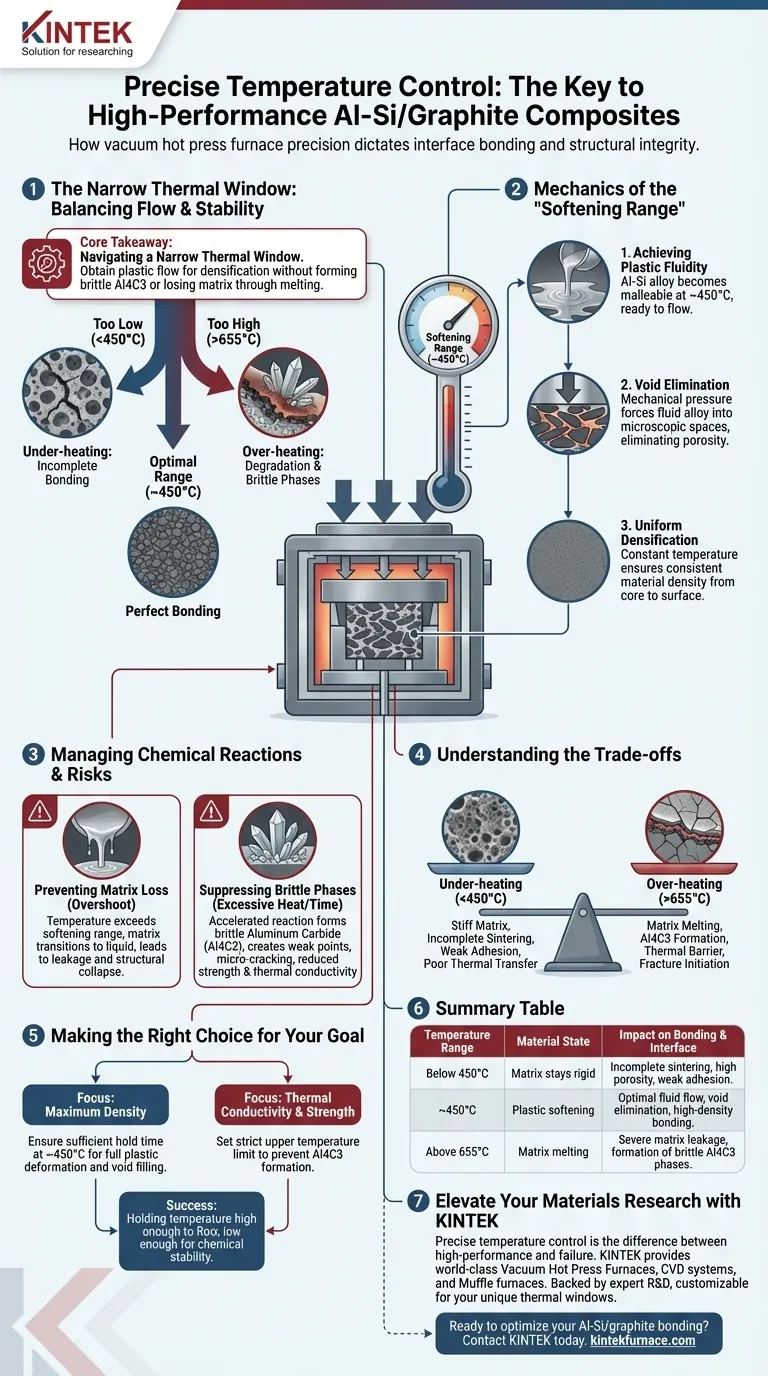
Precise temperature control dictates the structural integrity of the composite by maintaining the matrix in a specific "softening" state.
By stabilizing the process temperature within the softening range of the matrix material (approximately 450°C), the Aluminum-Silicon (Al-Si) alloy achieves the necessary plastic fluidity to physically fill voids between graphite flakes. This precision prevents the two extremes of failure: incomplete bonding due to stiffness, or the structural degradation caused by matrix melting and brittle phase formation.
Core Takeaway Obtaining a high-performance Al-Si/graphite composite requires navigating a narrow thermal window. You must apply enough heat to induce plastic flow for densification, while strictly capping the temperature to prevent the formation of brittle aluminum carbide (Al4C3) and the loss of matrix material through melting.

The Mechanics of the "Softening Range"
Achieving Plastic Fluidity
The primary objective of thermal regulation in this context is to reach the matrix's softening point. At approximately 450°C, the Al-Si alloy becomes malleable enough to flow under pressure.
Void Elimination
Once the matrix achieves this plastic state, the mechanical pressure of the hot press forces the alloy into the microscopic interstitial spaces between graphite flakes. This eliminates porosity and creates a continuous, high-density interface.
Uniform Densification
Using the vacuum hot press to hold this temperature constant ensures that the fluidity is uniform throughout the part. This promotes particle rearrangement and ensures the material density is consistent from the core to the surface.
Managing Chemical Reactions at the Interface
Preventing Matrix Loss
If the temperature control fails and overshoots the softening range, the matrix risks transitioning from a plastic solid to a liquid. This leads to matrix melting and leakage, effectively "starving" the composite of its binding agent and destroying the material's structure.
Suppressing Brittle Phases
The most critical chemical risk in this process is the formation of aluminum carbide (Al4C3). While a minimal amount of interfacial reaction can aid bonding, excessive temperatures (or prolonged heating) accelerate the reaction between Aluminum and Graphite.
The Impact of Al4C3
Al4C3 is a brittle reactant. If precise temperature control is not maintained and this phase grows excessively, the interface becomes a weak point. This leads to micro-cracking and a significant reduction in the composite's overall strength and thermal conductivity.
Understanding the Trade-offs
The Risk of Under-heating
If the temperature is too low (below the softening threshold), the Al-Si matrix remains too stiff. The applied pressure will not be sufficient to close voids, leading to incomplete sintering. The result is a porous material with weak mechanical adhesion and poor thermal transfer properties.
The Risk of Over-heating
If the temperature exceeds the optimal window (e.g., approaching or exceeding 655°C), you trade densification for degradation. You may achieve fully dense material, but the chemical composition of the interface will change. The resulting thick layer of brittle aluminum carbide acts as a thermal barrier and a fracture initiation site.
Making the Right Choice for Your Goal
To optimize your Al-Si/graphite composite, you must tailor your temperature profile to balance flow against reactivity.
- If your primary focus is Maximum Density: Ensure your hold time at the softening point (approx. 450°C) is sufficient to allow full plastic deformation and void filling before cooling.
- If your primary focus is Thermal Conductivity and Strength: set a strict upper temperature limit to prevent the formation of Al4C3, as these brittle reactants significantly impede heat transfer and reduce fracture toughness.
Success lies in holding the temperature high enough to flow, but low enough to remain chemically stable.
Summary Table:
| Temperature Range | Material State | Impact on Bonding & Interface |
|---|---|---|
| Below 450°C | Matrix stays rigid | Incomplete sintering, high porosity, and weak adhesion. |
| At ~450°C | Plastic softening | Optimal fluid flow, void elimination, and high-density bonding. |
| Above 655°C | Matrix melting | Severe matrix leakage and formation of brittle Al4C3 phases. |
Elevate Your Materials Research with KINTEK
Precise temperature control is the difference between a high-performance composite and a failed sintering process. KINTEK provides world-class laboratory equipment, including Vacuum Hot Press Furnaces, CVD systems, and Muffle furnaces, specifically designed for the delicate thermal windows required in advanced metallurgy.
Our systems are backed by expert R&D and are fully customizable to meet your unique processing needs. Whether you are aiming for maximum density or superior thermal conductivity, our technology ensures your materials achieve the perfect "softening" state without degradation.
Ready to optimize your Al-Si/graphite bonding? Contact us today to find your custom furnace solution.
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- How do vacuum and argon affect Ag-Ti2SnC composites? Optimize Sintering with Industrial Hot-Pressing Furnaces
- Why is the synchronous axial pressure function of a Spark Plasma Sintering (SPS) furnace essential for MgTiO3-CaTiO3?
- What are the benefits of the high vacuum environment in a vacuum hot pressing sintering furnace? Gain Maximum Density
- What are the classifications of hot pressing sintering furnaces based on the use environment? Explore Types for Optimal Material Processing
- How does the high-precision temperature control system of a sintering furnace influence nano-copper microstructure?
- What factors should be considered when choosing a vacuum press for metalworking? Optimize Your Investment for Precision and Efficiency
- What is the impact of grain structure on material properties in hot pressing vs. cold compacting and sintering? Optimize Your Powder Metallurgy Process
- What role does a hot-press sintering furnace play in Y2O3-YAM composite ceramics? Achieve 100% Density & Control Grains



















