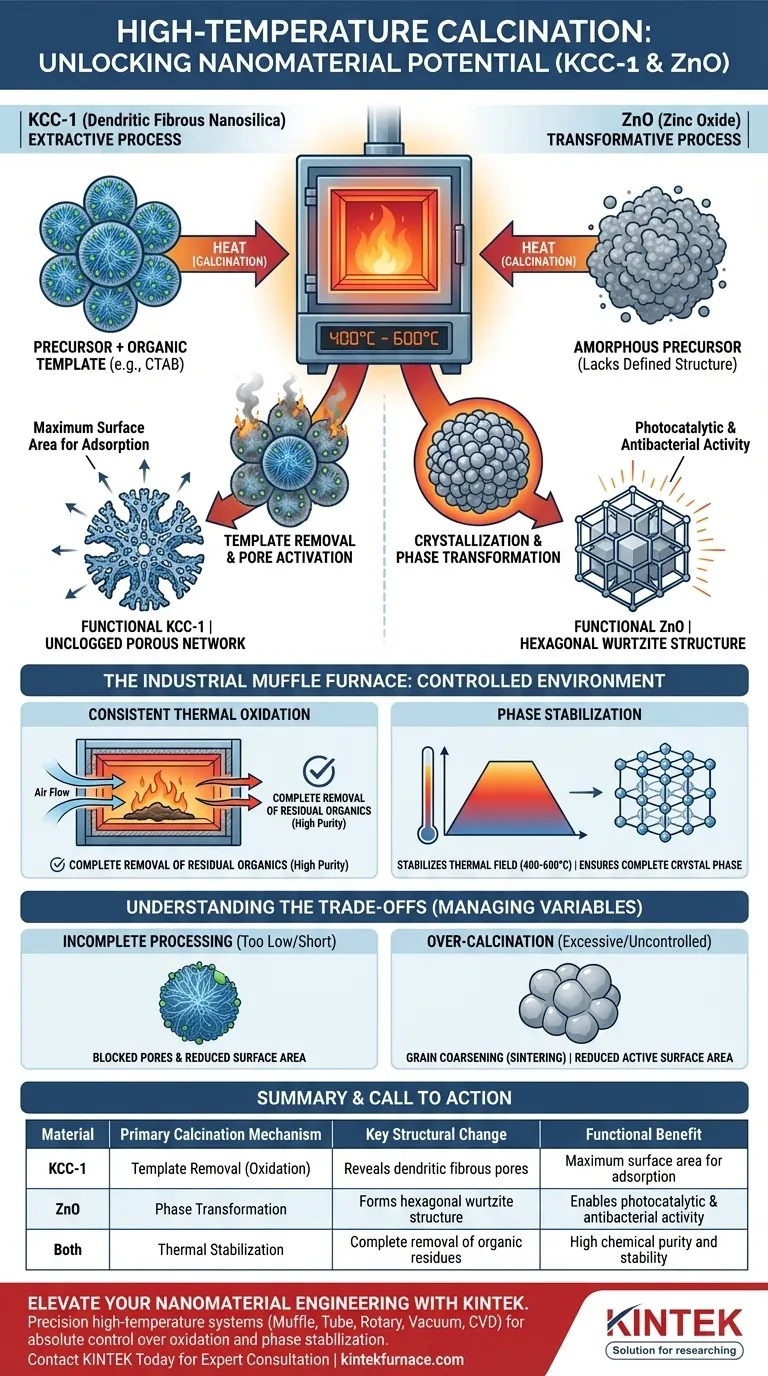
High-temperature calcination is the pivotal activation step that transforms raw chemical precursors into functional nanomaterials. In an industrial muffle furnace operating between 400°C and 600°C, this process drives essential chemical and structural changes—specifically, the removal of organic templates in KCC-1 and the crystallization of ZnO—to unlock their final performance capabilities.
Core Takeaway Calcination acts as a divergent functionalization tool depending on the material's requirement. For KCC-1, it is an extractive process designed to remove scaffolding and reveal surface area; for ZnO, it is a transformative process that converts amorphous compounds into a specific crystalline structure required for chemical reactivity.

The Mechanism of Functionalization
The utility of a nanopowder is defined by its surface architecture and crystal lattice. High-temperature calcination modifies these attributes through two primary mechanisms.
KCC-1: Template Removal and Pore Activation
For KCC-1 (Dendritic Fibrous Nanosilica), the primary goal of calcination is purification and void creation.
During synthesis, KCC-1 creates its unique fibrous shape around an organic template agent, typically CTAB (Cetyltrimethylammonium bromide).
Calcination burns away this organic template completely.
By removing the CTAB, the process releases the characteristic porous structure of the silica. Without this thermal extraction, the pores would remain blocked, rendering the material useless for applications requiring high surface area.
ZnO: Crystallization and Phase Transformation
For Zinc Oxide (ZnO), calcination is a process of structural ordering.
The raw precursor for ZnO is often an amorphous substance lacking a defined geometric arrangement.
The heat triggers thermal decomposition and subsequent crystallization.
This transforms the amorphous material into a highly stable hexagonal wurtzite structure.
This specific crystal phase is essential because it endows the ZnO with its functional properties, specifically its photocatalytic and antibacterial activities.
The Role of the Industrial Muffle Furnace
The industrial muffle furnace provides the controlled environment necessary to ensure these reactions reach completion without compromising material integrity.
Consistent Thermal Oxidation
The furnace maintains a continuous high-temperature air environment, essential for the thermal oxidation reaction.
This ensures the complete removal of residual organics, such as surfactants or solvents used during synthesis.
Any remaining organic residue can act as an impurity, degrading the performance of the final powder.
Phase Stabilization
Achieving the correct crystal phase requires precise thermal energy.
The furnace stabilizes the thermal field at 400–600°C, providing the energy barrier needed to transition from an amorphous state to a crystalline state.
This promotes complete crystal phase transformation, ensuring the final powder is chemically stable and reactive.
Understanding the Trade-offs
While high temperatures are necessary, they introduce variables that must be strictly managed to avoid degrading the nanomaterials.
The Risk of Incomplete Processing
If the temperature is too low or the duration too short, organic templates (like CTAB in KCC-1) may not burn off completely.
This results in blocked pores and reduced surface area, significantly hampering the material's adsorption capabilities.
The Risk of Over-Calcination
Conversely, excessive heat or lack of control can lead to negative structural changes.
In crystalline materials, uncontrolled heat can cause grain coarsening or sintering.
This reduces the active surface area of the powder, potentially diminishing the very reactivity (such as photocatalytic activity in ZnO) that the process was meant to create.
Making the Right Choice for Your Goal
To maximize the functionality of your nanopowders, align your processing parameters with your specific material objectives.
- If your primary focus is KCC-1 (High Surface Area): Prioritize complete oxidation of the organic template to fully unclog the porous network.
- If your primary focus is ZnO (Reactivity): Prioritize reaching the specific temperature window (400–600°C) that guarantees the formation of the hexagonal wurtzite phase.
Successful functionalization relies not just on heating the material, but on using heat to engineer the precise atomic architecture required for the application.
Summary Table:
| Material | Primary Calcination Mechanism | Key Structural Change | Functional Benefit |
|---|---|---|---|
| KCC-1 | Template Removal (Oxidation) | Reveals dendritic fibrous pores | Maximum surface area for adsorption |
| ZnO | Phase Transformation | Forms hexagonal wurtzite structure | Enables photocatalytic & antibacterial activity |
| Both | Thermal Stabilization | Complete removal of organic residues | High chemical purity and stability |
Elevate Your Nanomaterial Engineering with KINTEK
Precision at high temperatures is the difference between a failed precursor and a functional nanomaterial. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to give you absolute control over thermal oxidation and phase stabilization.
Whether you are unclogging the porous network of KCC-1 or engineering the crystal lattice of ZnO, our customizable lab furnaces ensure consistent thermal fields and reliable results tailored to your unique research needs.
Ready to optimize your calcination process?
Contact KINTEK Today for a Expert Consultation
Visual Guide
References
- Farzaneh Edrisi, Nasrin Shadjou. Preparation of an innovative series of respiratory nano-filters using polystyrene fibrous films containing KCC-1 dendrimer and ZnO nanostructures for environmental assessment of SO<sub>2</sub>, NO<sub>2</sub> and CO<sub>2</sub>. DOI: 10.1039/d4ra00176a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What advantages do rapid heating and cooling features offer in some muffle furnace models? Boost Efficiency and Control in Your Lab
- What industrial applications do muffle furnaces have in heat treating? Precision Solutions for High-Tech Industries
- What is the primary function of an industrial box furnace? Master 60Si2CrV Spring Steel Heat Treatment
- Why Use a High-Temp Muffle Furnace for Al–Ce–La–Ni–Fe Alloys? Ensure Thermal Stability with Precision Air-Cooling
- What are the applications of muffle furnaces? Essential for High-Temperature Material Processing
- What is a muffle furnace and what is its primary function? Discover Its Role in High-Temperature Processing
- What is the primary function of a muffle furnace during carbonization? Master Coffee-Based Bio-Adsorbent Production
- What role does an industrial microwave muffle furnace play in the sintering process of porous mullite ceramic skeletons?



















