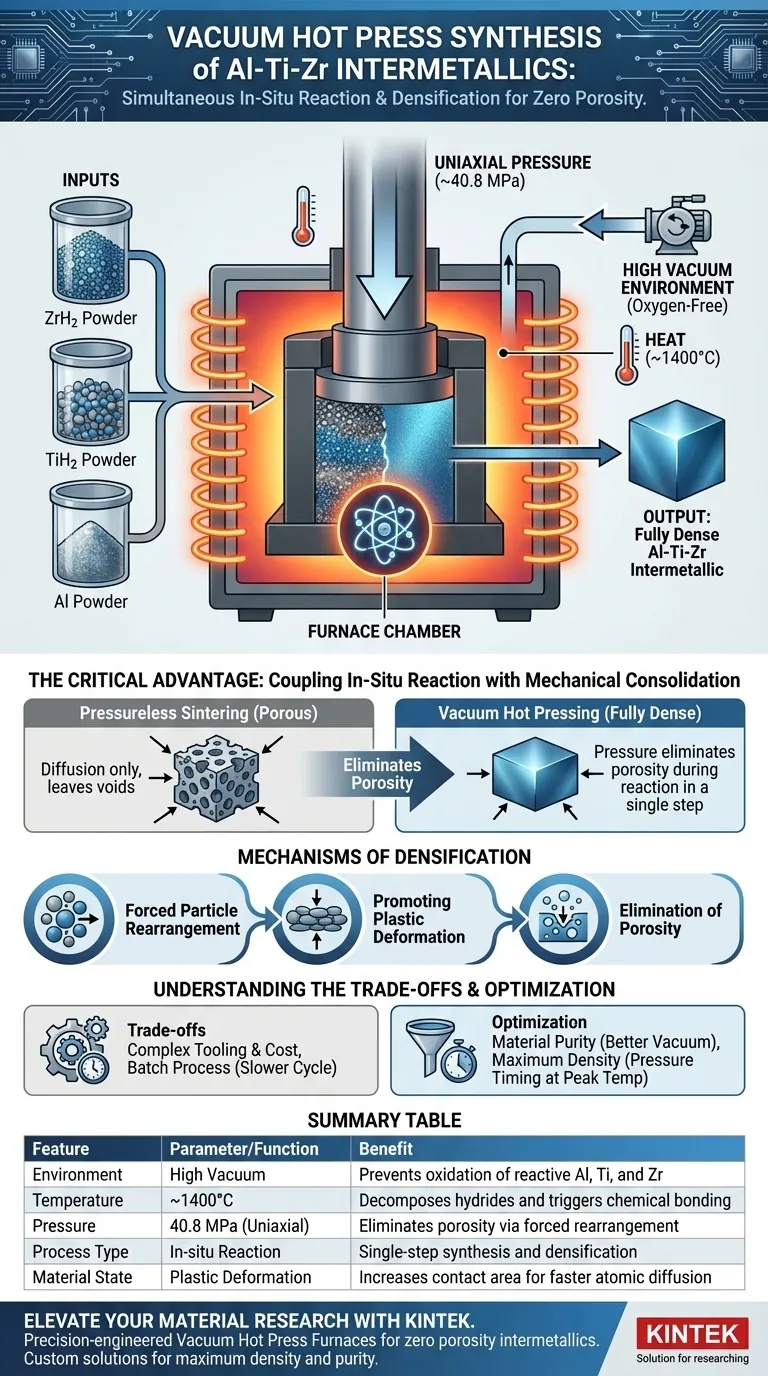
A Vacuum Hot Press Furnace facilitates the synthesis of Al-Ti-Zr compounds by subjecting precursor powders to simultaneous high thermal energy and uniaxial mechanical pressure within an oxygen-free environment. Specifically, it heats ZrH2, TiH2, and Al powders to approximately 1400°C to trigger chemical reactions while applying roughly 40.8 MPa of pressure to mechanically force densification.
The critical advantage of this technology is the coupling of in-situ reaction with mechanical consolidation. By applying pressure during the chemical synthesis phase, the furnace eliminates the porosity that naturally occurs during reaction, yielding a fully dense material in a single step.

Creating a Controlled Reaction Environment
The Necessity of Vacuum Protection
Aluminum, Titanium, and Zirconium are highly reactive metals with strong affinities for oxygen.
The furnace’s vacuum atmosphere is the first line of defense. It removes oxygen from the chamber, effectively preventing the oxidation of the metal powders during the heating ramp.
Triggering In-Situ Reactions
The process utilizes hydride powders (ZrH2 and TiH2) mixed with Aluminum rather than pure elemental metals.
The high-temperature environment (reaching 1400°C) provides the necessary activation energy to decompose these hydrides. This decomposition releases reactive metal species that immediately bond with the aluminum to synthesize the target Al-Ti-Zr ternary intermetallic compound.
Mechanisms of Densification
Forced Particle Rearrangement
In standard sintering, densification relies on diffusion, which can be slow and leave voids.
The Vacuum Hot Press introduces significant mechanical pressure (e.g., 40.8 MPa). This physical force mechanically pushes the powder particles past one another, filling large interstitial gaps before diffusion even begins.
Promoting Plastic Deformation
As the temperature rises, the yield strength of the material decreases.
The applied uniaxial pressure causes the particles to undergo plastic deformation at these contact points. This deformation flattens the particles against each other, significantly increasing the contact area available for atomic diffusion.
Elimination of Porosity
Chemical reactions often result in volume changes that create internal porosity.
By maintaining high pressure throughout the reaction phase, the furnace actively collapses these voids as they form. This results in a bulk material with near-theoretical density, far superior to what pressureless sintering could achieve.
Understanding the Trade-offs
Equipment Complexity and Cost
While the results are superior, the process requires complex tooling, typically graphite dies, which must withstand both extreme heat and high pressure.
This increases the operational cost and limits the geometric complexity of the parts you can produce compared to standard sintering or casting.
Cycle Time Limitations
Hot pressing is inherently a batch process.
Because the synthesis and densification occur simultaneously under pressure, the system must undergo full heating and cooling cycles for each load. This generally results in lower throughput compared to continuous sintering furnaces.
Making the Right Choice for Your Goal
To maximize the efficacy of a Vacuum Hot Press for Al-Ti-Zr compounds, align your process parameters with your specific material requirements:
- If your primary focus is Material Purity: Ensure your vacuum system is capable of maintaining high-vacuum levels throughout the hydride decomposition phase to prevent getter effects.
- If your primary focus is Maximum Density: Prioritize the timing of the pressure application (40.8 MPa) to coincide exactly with the peak reaction temperature (1400°C) to collapse pores while the material is most malleable.
Ultimately, the Vacuum Hot Press is the definitive tool when material integrity and density cannot be compromised by residual porosity.
Summary Table:
| Feature | Parameter/Function | Benefit in Synthesis |
|---|---|---|
| Environment | High Vacuum | Prevents oxidation of reactive Al, Ti, and Zr |
| Temperature | ~1400°C | Decomposes hydrides and triggers chemical bonding |
| Pressure | 40.8 MPa (Uniaxial) | Eliminates porosity via forced particle rearrangement |
| Process Type | In-situ Reaction | Single-step synthesis and densification |
| Material State | Plastic Deformation | Increases contact area for faster atomic diffusion |
Elevate Your Material Research with KINTEK
Are you looking to synthesize advanced intermetallics with zero porosity? KINTEK’s precision-engineered Vacuum Hot Press Furnaces provide the ultimate control over thermal and mechanical parameters needed for complex ternary compounds like Al-Ti-Zr.
Backed by expert R&D and world-class manufacturing, we offer a full range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to your specific laboratory or industrial requirements. Our systems ensure your materials achieve maximum density and purity every time.
Ready to optimize your synthesis process? Contact our technical experts today to discuss a custom high-temperature solution for your unique needs!
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- Why is maintaining a high vacuum environment essential during the hot pressing of aluminum-based laminated composites? Ensure Superior Bonding and Density
- What are the advantages of a vacuum hot pressing sintering furnace for rare earth copper composites? Density & Purity
- How does the pressurization system of a vacuum hot press affect SiC/TB8 composites? Optimize Matrix Densification
- What are the different pressurization methods used in vacuum hot pressing? Choose Uniaxial vs. HIP for Your Lab
- What role does a high-pressure press play in the preparation of zinc sample pellets? Optimize Carbothermic Reduction
- How does hot-press sintering contribute to manufacturing high-density Ta-Al-C MAX phase ceramics? Optimize Consolidation
- Why are hot press furnaces important in materials research? Unlock Advanced Material Synthesis
- What technical advantages does a Spark Plasma Sintering (SPS) system offer for TiB2 ceramics? Unlock Superior Strength



















