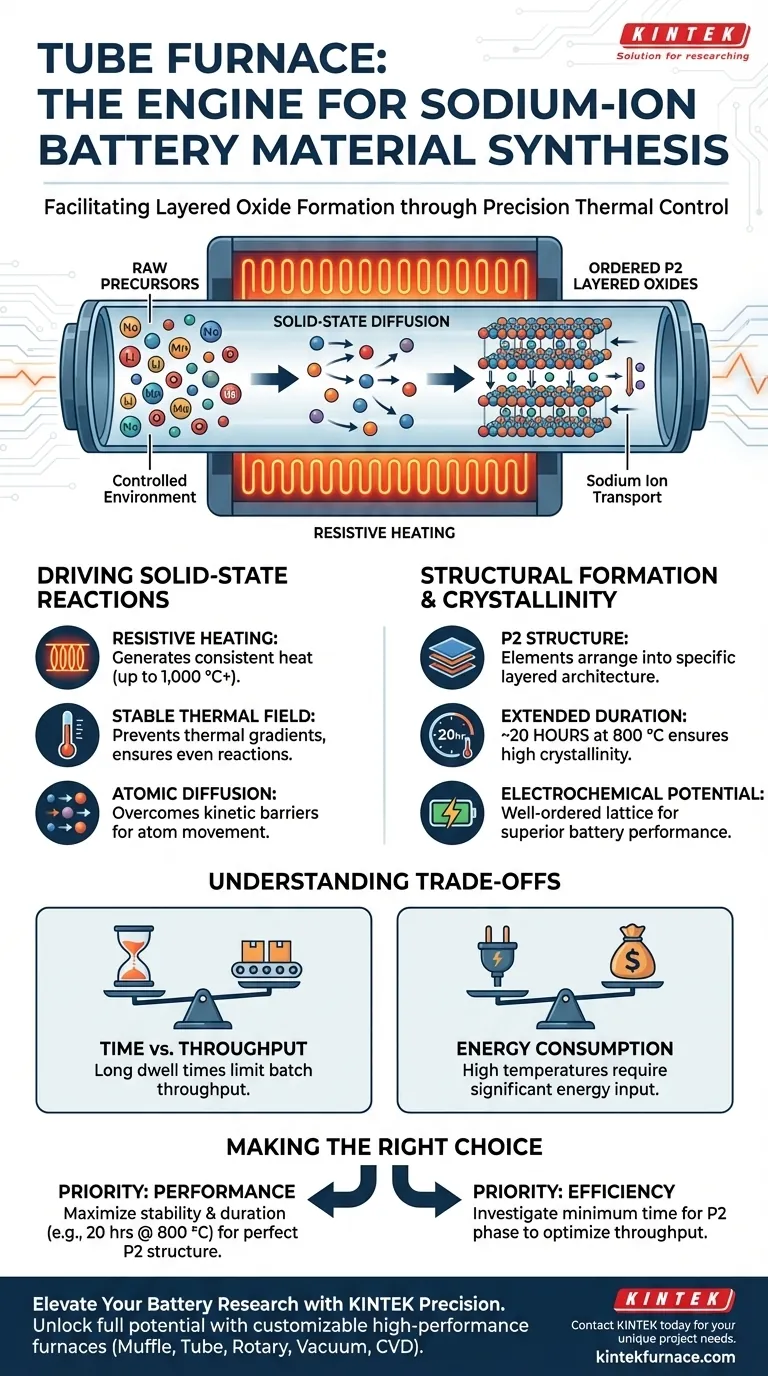
A tube furnace functions as a precision thermal vessel designed to drive the complex solid-state reactions required for sodium-ion battery materials. By generating a highly stable thermal field—often maintained at 800 °C for durations exceeding 20 hours—it facilitates the atomic diffusion necessary to transform raw precursors into ordered, high-performance layered oxides.
The tube furnace is not merely a heat source; it is a structural enabler. By providing a controlled environment for solid-state diffusion, it allows constituent elements to arrange into the specific P2-type layered architectures that define high-quality, crystalline cathode materials.

Driving Solid-State Reactions
The Mechanics of Heating
A tube furnace operates using resistive heating elements that surround the central tube. These elements generate consistent heat, allowing the interior chamber to reach and maintain precise temperatures ranging from a few hundred degrees to over 1,000 °C.
Facilitating Atomic Diffusion
The synthesis of layered oxides, such as Sodium-Lithium-Manganese-Oxide (NLMO), relies heavily on solid-state diffusion. This process requires atoms to move through a solid lattice to form new compounds, a mechanism that is kinetically slow at room temperature.
The Role of Thermal Stability
To overcome these kinetic barriers, the furnace provides a stable thermal field. Maintaining a constant temperature prevents thermal gradients that could lead to uneven reaction rates or inconsistent material properties across the sample batch.
Structural Formation and Crystallinity
Achieving the P2 Structure
The primary goal of this thermal treatment is structural organization. Under these specific thermal conditions, the elements rearrange themselves into an ordered P2 structure, a specific layering arrangement critical for sodium-ion transport.
The Importance of Duration
Time is as critical as temperature. The primary reference notes that maintaining 800 °C for extended periods, such as 20 hours, is necessary. This duration ensures the reaction creates a material with high crystallinity, rather than an amorphous or poorly ordered solid.
Enhancing Electrochemical Potential
The high crystallinity resulting from this controlled synthesis directly correlates to the material's performance. A well-ordered crystal lattice allows for more efficient sodium ion movement, resulting in superior electrochemical activity in the final battery cell.
Understanding the Trade-offs
Processing Time vs. Throughput
The synthesis process is inherently time-intensive. Dedicating a furnace to a single batch for 20+ hours (excluding ramp-up and cool-down times) limits immediate throughput, making this a batch-process bottleneck.
Energy Consumption
Maintaing high temperatures (800 °C) for nearly a full day requires significant energy input. This cost is necessary to achieve the high crystallinity required for top-tier battery performance but impacts the overall energy efficiency of the manufacturing process.
Making the Right Choice for Your Goal
To optimize your synthesis strategy, consider your specific material requirements:
- If your primary focus is electrochemical performance: Prioritize the stability and duration of the heat treatment (e.g., 20 hours at 800 °C) to maximize crystallinity and ensure a perfect P2 structure.
- If your primary focus is process efficiency: Investigate the minimum time required to achieve the P2 phase, as excessive heating beyond the point of crystallization yields diminishing returns.
The tube furnace is the critical instrument for converting raw chemical potential into the structured, crystalline reality required for modern energy storage.
Summary Table:
| Feature | Role in Synthesis | Benefit for Battery Materials |
|---|---|---|
| Precise Thermal Field | Maintains stable 800 °C environments | Prevents thermal gradients and uneven reactions |
| Extended Dwell Time | 20+ hours of continuous heating | Ensures high crystallinity and complete atomic diffusion |
| Resistive Heating | Controlled energy distribution | High-purity transformation of raw precursors |
| Structural Control | Facilitates P2-type layering | Optimizes sodium-ion transport and electrochemical activity |
Elevate Your Battery Research with KINTEK Precision
Unlock the full potential of your energy storage materials with KINTEK’s industry-leading thermal solutions. Backed by expert R&D and world-class manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the rigorous demands of sodium-ion battery synthesis. Whether you are optimizing P2-type layered oxides or developing next-generation crystalline structures, our furnaces provide the stability and control your lab requires.
Ready to achieve superior material crystallinity? Contact KINTEK today to discuss your unique project needs.
Visual Guide
References
- Junhua Zhou, Mark H. Rümmeli. Titanium Substitution Facilitating Oxygen and Manganese Redox in Sodium Layered Oxide Cathode. DOI: 10.1002/admi.202400190
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the role of a Tube Furnace in TMDC-ND preparation? Master Graphene-Decorated Nanostructure Synthesis
- Why Use Nitrogen in Lab Tube Furnaces for LFP Relithiation? Ensure Superior Battery Material Restoration
- How does a fluidized bed vertical tube furnace work? Achieve Superior Heating Efficiency and Uniformity
- How does a high-temperature tube furnace facilitate the synthesis of Ni17W3/MoO3-x/WO3-x catalysts during annealing?
- What are the advantages of vertical tube furnaces? Achieve Precision and Efficiency in Your Lab
- How does a horizontal dual-zone tube furnace facilitate WSe2 CVT growth? Precision Thermal Gradient Control
- What is the application of a high-temperature tube resistance furnace in studying HEA coatings? | KINTEK
- How does the heating method of a fluidized bed vertical tube furnace differ from ordinary tube furnaces? Discover Key Differences for Better Lab Efficiency



















