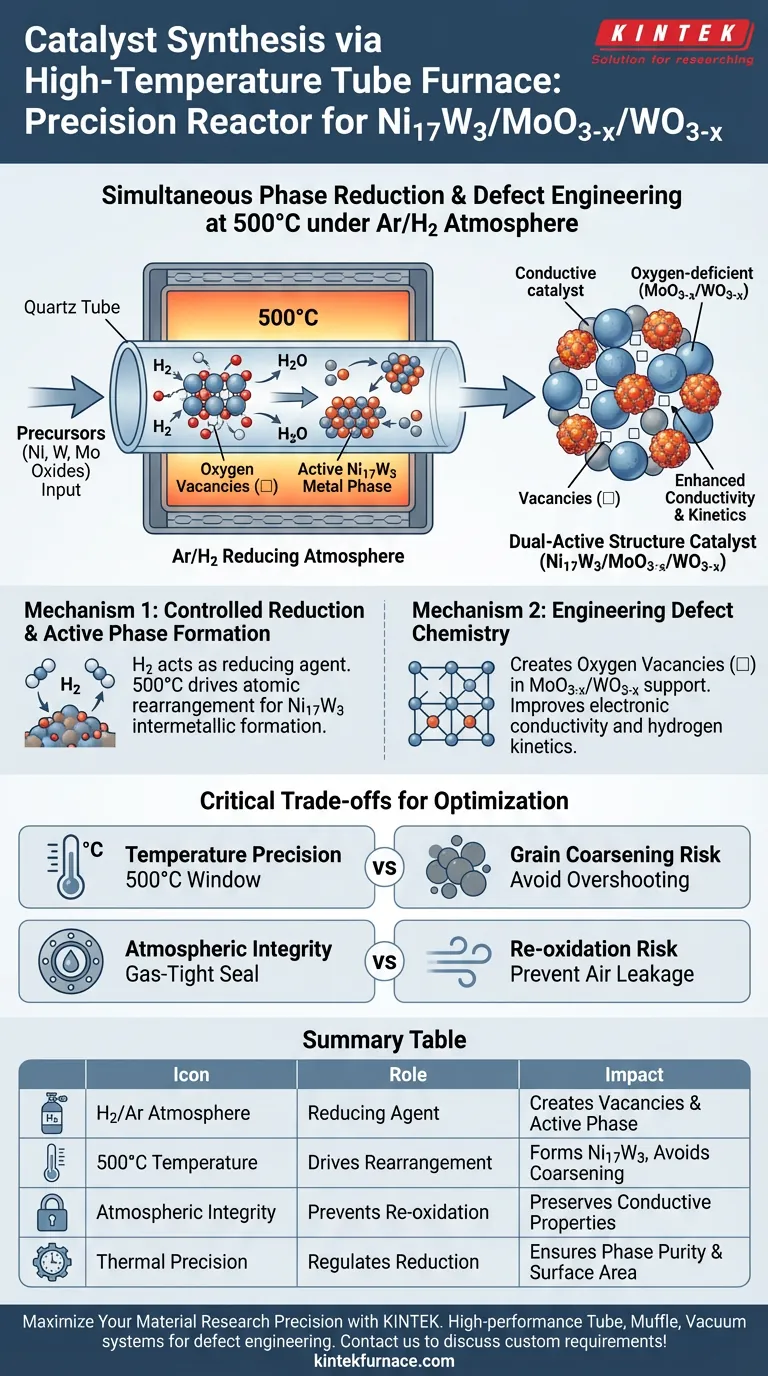
The high-temperature tube furnace serves as a precision reactor that drives simultaneous phase reduction and defect engineering. Specifically, it maintains a 500°C environment under a reducing Argon/Hydrogen (Ar/H2) atmosphere. This controlled setting is required to transform precursors into the active Ni17W3 metal phase while inducing specific chemical defects in the supporting oxides.
Core Takeaway The tube furnace does not merely heat the material; it utilizes a reducing H2 atmosphere to strip oxygen atoms from the lattice, creating critical oxygen vacancies. This process creates a dual-active structure: a highly conductive, oxygen-deficient support (MoO3-x/WO3-x) anchored with the active Ni17W3 metal phase.
The Mechanism of Controlled Reduction
Precise Atmosphere Regulation
The synthesis relies heavily on the furnace's ability to maintain a strictly controlled reducing atmosphere, specifically an Ar/H2 mixture.
Unlike simple heating, the presence of hydrogen gas actively participates in the chemical reaction. It acts as a reducing agent that is essential for converting the initial precursors into the desired metallic and sub-oxide states.
Formation of the Active Metal Phase
Under these 500°C conditions, the furnace drives the reduction of metal precursors into the specific Ni17W3 phase.
This phase is the "active" component of the catalyst. The thermal energy provided by the furnace ensures the atomic rearrangement necessary for nickel and tungsten to form this specific intermetallic compound, rather than remaining as separate oxides.
Engineering Defect Chemistry
Inducing Oxygen Vacancies
A critical function of the furnace is the creation of oxygen vacancies within the metal oxide support, denoted as MoO3-x and WO3-x.
By controlling the reduction depth, the process removes specific oxygen atoms from the crystal lattice. These missing atoms (vacancies) are not defects in a negative sense; they are engineered features that dramatically alter the material's electronic structure.
Enhancing Conductivity and Kinetics
The introduction of these vacancies directly improves the material's electronic conductivity.
Furthermore, these structural gaps enhance hydrogen insertion and extraction kinetics. The furnace environment ensures that these vacancies are distributed abundantly, optimizing the catalyst for electrochemical performance.
Understanding the Trade-offs
Temperature Precision vs. Grain Coarsening
While high temperatures are necessary for phase conversion, excessive heat can be detrimental.
If the temperature exceeds the optimal 500°C window, there is a risk of grain coarsening (atomic clumping), which reduces the active surface area. The tube furnace must offer precise programmable control to prevent "overshooting" the target temperature.
Atmospheric Integrity
The effectiveness of vacancy creation is entirely dependent on the furnace's sealing integrity.
Any leakage of ambient air (oxygen) into the tube during the annealing process will re-oxidize the material, filling the vacancies and destroying the conductive properties you are trying to engineer. The stability of the reducing atmosphere is as critical as the temperature itself.
Making the Right Choice for Your Goal
To optimize the synthesis of Ni17W3/MoO3-x/WO3-x catalysts, consider these operational priorities:
- If your primary focus is maximizing electronic conductivity: Ensure your gas flow rates maintain a consistent H2 concentration to maximize oxygen vacancy formation in the support.
- If your primary focus is phase purity: Prioritize the precision of the thermal ramp and dwell time at 500°C to ensure complete precursor reduction without inducing thermal degradation.
The tube furnace is the tool that transforms a chemical mixture into a functional catalyst by strictly enforcing the boundary between reduction and oxidation.
Summary Table:
| Process Component | Role in Catalyst Synthesis | Impact on Material Performance |
|---|---|---|
| H2/Ar Atmosphere | Acts as a reducing agent to strip oxygen atoms | Creates critical oxygen vacancies and active metal phases |
| 500°C Temperature | Drives atomic rearrangement and precursor conversion | Forms Ni17W3 intermetallic compound without grain coarsening |
| Atmospheric Integrity | Prevents re-oxidation from ambient air leakage | Preserves conductive properties and engineered structural defects |
| Thermal Precision | Regulates reduction depth and dwell time | Ensures phase purity and maximizes active surface area |
Maximize Your Material Research Precision with KINTEK
Precise control over atmosphere and temperature is non-negotiable for defect engineering and catalyst synthesis. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems designed to meet the most rigorous laboratory standards. Whether you are synthesizing advanced Ni17W3 catalysts or developing next-generation energy materials, our customizable high-temperature furnaces provide the atmospheric integrity and thermal stability your research demands.
Ready to elevate your lab's capabilities? Contact KINTEK today to discuss your custom furnace requirements!
Visual Guide
References
- Yiqing Sun, Xianying Wang. Oxygen vacancy-induced efficient hydrogen spillover in Ni<sub>17</sub>W<sub>3</sub>/WO<sub>3−<i>x</i></sub>/MoO<sub>3−<i>x</i></sub> for a superior pH-universal hydrogen evolution reaction. DOI: 10.1039/d4ta00729h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the primary role of a Tube Furnace in g-C3N4 synthesis? Achieve Precise Thermal Polycondensation
- Why is a silicate glass fixed-bed reactor used instead of stainless steel? Ensure Pure Methanol Decomposition Data
- What is the function of Argon gas flow within a Tube Furnace during the heat treatment of Molybdenum Disulfide? Expert Guide
- What is the function of quartz vacuum encapsulation in RhSeCl CVT? Mastering Pure Crystal Growth
- What temperature control features do tube turnouts typically have? Achieve Precise Thermal Management for Your Lab
- How does a high-precision tube furnace influence the growth quality of graphene? Optimize CVD Synthesis Performance
- What physical conditions does a tube furnace provide for biomass pyrolysis? Master Thermal Control for Bio-Energy
- What is the significance of a vacuum tube furnace system? Master Reaction Rate Constants for Carbonate Thin Films



















