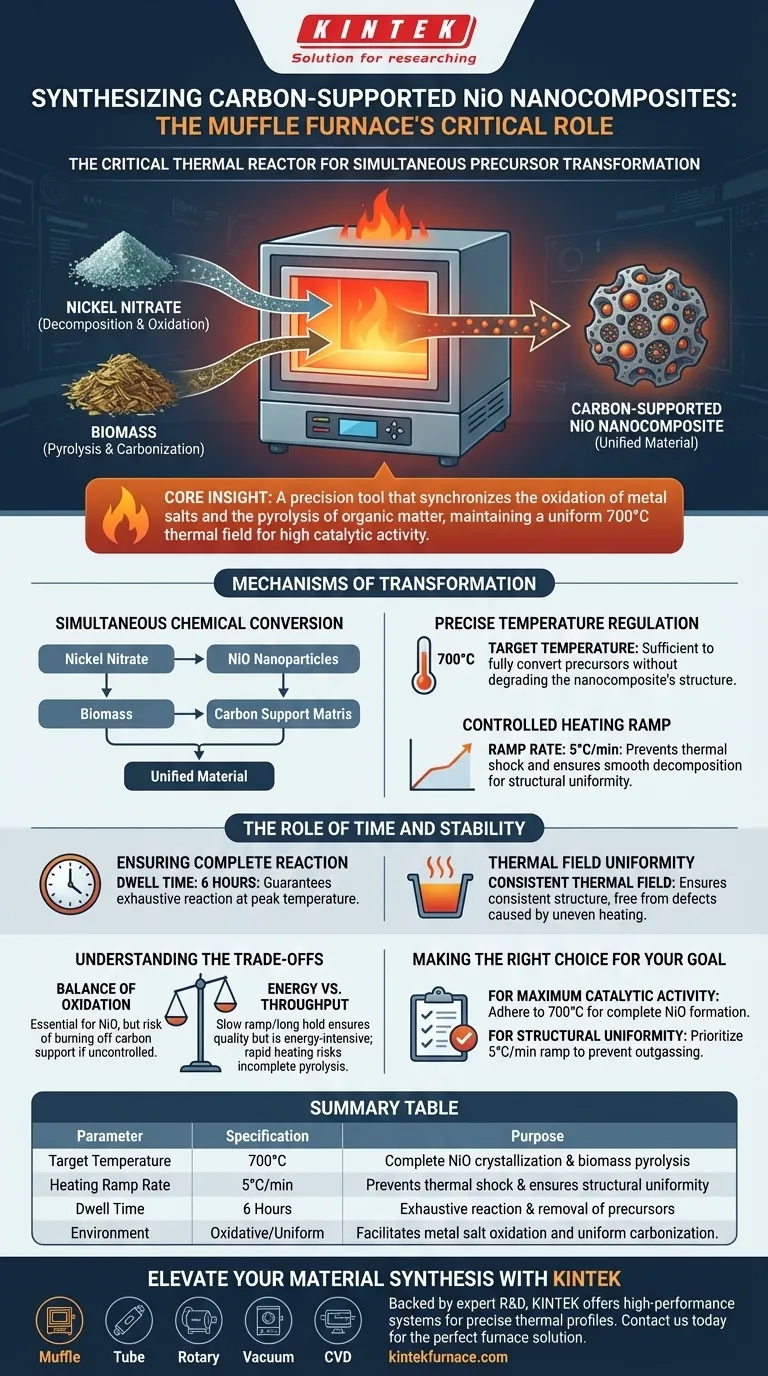
The muffle furnace acts as the critical thermal reactor required to simultaneously drive the chemical transformation of two distinct precursors. It provides a stable, high-temperature environment that facilitates the decomposition of nickel nitrate into nickel oxide (NiO) while concurrently converting biomass into a robust carbon support structure.
Core Insight: The muffle furnace is not merely a heating element; it is a precision tool that synchronizes the oxidation of metal salts and the pyrolysis of organic matter. Its ability to maintain a uniform thermal field at 700°C ensures the resulting nanocomposite achieves the high catalytic activity necessary for performance.

Mechanisms of Transformation
Simultaneous Chemical Conversion
The primary function of the muffle furnace in this synthesis is to manage two chemical reactions at once.
First, it drives the complete decomposition and oxidation of nickel nitrate.
Second, it induces the thorough pyrolysis of the biomass precursor. This dual action results in a unified material where NiO nanoparticles are embedded within a carbon matrix.
Precise Temperature Regulation
Achieving the correct crystalline phase requires exacting temperature control.
The furnace is programmed to reach a target temperature of 700°C.
This specific thermal point is sufficient to fully convert the precursors without degrading the structural integrity of the newly formed nanocomposite.
Controlled Heating Ramp
The rate at which temperature increases is as important as the final temperature.
The muffle furnace utilizes a ramp rate of 5°C per minute.
This slow, steady increase prevents thermal shock and ensures that the decomposition of organic components is smooth, leading to a structurally uniform material.
The Role of Time and Stability
Ensuring Complete Reaction
Thermal synthesis is time-dependent.
The process requires holding the peak temperature of 700°C for a duration of 6 hours.
This extended dwell time guarantees that the conversion of nickel nitrate to NiO is exhaustive, leaving no unreacted precursors behind.
Thermal Field Uniformity
Beyond simple heating, the muffle furnace provides a consistent thermal field.
This uniformity ensures that the material at the center of the crucible experiences the exact same conditions as the material at the edges.
The result is a coarse carbon precursor with a consistent structure, free from the defects caused by uneven heating gradients.
Understanding the Trade-offs
The Balance of Oxidation
The muffle furnace provides an oxidative environment, which is essential for forming Nickel Oxide (NiO).
However, this presents a delicate balance when dealing with carbon.
If the temperature or oxygen exposure becomes uncontrolled, there is a risk of burning off the carbon support entirely, leaving only metal oxide ash.
Energy vs. Throughput
The heating profile defined—specifically the slow ramp and long hold—is energy-intensive.
While a 5°C/min ramp ensures high quality and uniformity, it significantly extends the total processing time.
Rapid heating might save time but often leads to incomplete pyrolysis or structural collapse of the carbon framework.
Making the Right Choice for Your Goal
To optimize the synthesis of NiO nanocomposites, tailor your furnace settings to your specific objectives:
- If your primary focus is Maximum Catalytic Activity: Adhere strictly to the 700°C target temperature to ensure the complete formation of the active NiO phase.
- If your primary focus is Structural Uniformity: Prioritize the 5°C/min ramp rate to prevent rapid outgassing and ensure a smooth, even carbonization of the biomass.
By controlling the thermal environment with precision, you transform raw biomass and salts into a sophisticated, high-performance catalyst.
Summary Table:
| Parameter | Specification | Purpose in Synthesis |
|---|---|---|
| Target Temperature | 700°C | Ensures complete NiO crystallization & biomass pyrolysis |
| Heating Ramp Rate | 5°C/min | Prevents thermal shock & ensures structural uniformity |
| Dwell Time | 6 Hours | Guarantees exhaustive reaction & removal of precursors |
| Environment | Oxidative/Uniform | Facilitates metal salt oxidation and uniform carbonization |
Elevate Your Material Synthesis with KINTEK
Precision is the difference between a failed reaction and a high-performance catalyst. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to deliver the exact thermal profiles required for complex nanocomposite synthesis. Whether you are scaling up biomass conversion or optimizing lab-scale R&D, our customizable high-temperature furnaces provide the uniformity and control your research demands.
Ready to achieve superior catalytic activity? Contact us today to find the perfect furnace solution for your lab.
Visual Guide
References
- Sunshine D. Kurbah, Ndege Simisi Clovis. Lignocellulosic Biomass Derived Carbon Supported Nickel Nanoparticles as an Efficient Catalyst for Reduction of Nitroarenes. DOI: 10.17807/orbital.v16i4.21957
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What optional features are available for box furnaces? Customize for Your Lab's Unique Needs
- What is the primary technical function of a Muffle Furnace in CoMn2O4 synthesis? Achieve Precise Nano-Spinel Calcination
- What are the installation and maintenance benefits of electric furnaces? Achieve Simpler, Lower-Cost Heating
- What role does an electric muffle furnace play in the siliconization of 10Kh23N18 steel welds? Expert Thermal Insight
- What temperature ranges can muffle furnaces achieve? Find the Perfect Heat for Your Lab Needs
- What is the function of a high-temperature muffle furnace in the solid-state synthesis of CaBiO2Cl? Expert Insights
- What is the function of a Muffle Furnace in the LSS process for MXene synthesis? Achieve Low-Temp Precision
- What safety features are incorporated in muffle furnace designs? Ensure Operator and Equipment Protection



















