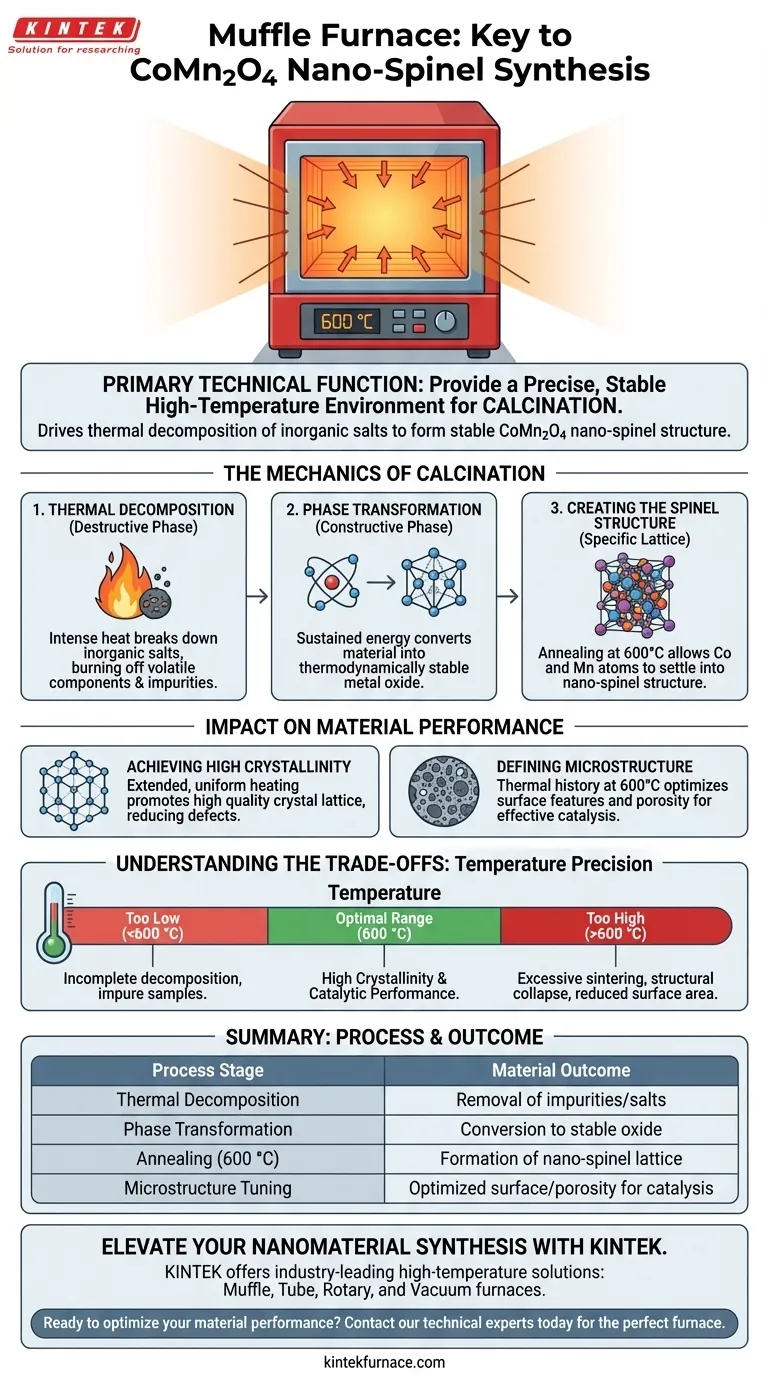
The primary technical function of a Muffle Furnace in this synthesis is to provide a precise, stable high-temperature environment for calcination. Specifically, by maintaining a temperature of approximately 600 °C, the furnace drives the thermal decomposition of inorganic salt precursors. This converts them into the stable metal oxide crystal phases necessary to form the final CoMn2O4 nano-spinel structure.
By controlling the thermal environment, the Muffle Furnace bridges the gap between raw chemical precursors and functional materials. It facilitates the atomic rearrangement required to achieve high crystallinity and the specific microstructures essential for effective catalysis.

The Mechanics of Calcination
Thermal Decomposition
The initial role of the furnace is destructive. It subjects the precursor materials to intense heat to break down inorganic salts. This step effectively burns off volatile components and impurities that are leftovers from the co-precipitation stage, leaving behind only the desired metal species.
Phase Transformation
Once impurities are removed, the sustained heat drives a constructive phase transformation. The furnace provides the energy required for the remaining metal atoms to rearrange themselves. This rearrangement converts the material from a precursor state into a thermodynamically stable metal oxide.
Creating the Spinel Structure
For CoMn2O4, this is not just about forming an oxide; it is about achieving a specific crystal lattice. The annealing process allows the cobalt and manganese atoms to settle into the complex nano-spinel structure. Without this controlled heating, the material would likely remain amorphous or settle into unwanted phases.
Impact on Material Performance
Achieving High Crystallinity
The duration and stability of the heat treatment directly dictate the quality of the crystal lattice. A Muffle Furnace ensures that the material is heated evenly for several hours. This extended exposure promotes high crystallinity, reducing defects that could hinder electron transport or structural integrity.
Defining Microstructure
The thermal history of the sample determines its physical morphology. The specific annealing parameters (600 °C) are tuned to produce microstructures optimized for catalysis. The furnace ensures the material develops the surface features and porosity required to interact effectively with other chemicals in catalytic applications.
Understanding the Trade-offs
Temperature Precision vs. Material Integrity
While high heat is necessary, temperature control is the critical variable. If the temperature is too low, the decomposition of inorganic salts will be incomplete, resulting in impure samples. Conversely, if the temperature exceeds the optimal 600 °C range, you risk excessive sintering. This can cause the nano-structures to collapse into larger bulk crystals, drastically reducing the active surface area required for catalysis.
Making the Right Choice for Your Goal
To maximize the effectiveness of the CoMn2O4 synthesis, you must tailor the furnace parameters to your specific objectives.
- If your primary focus is Chemical Purity: Ensure the furnace maintains the target temperature (600 °C) long enough to guarantee the total decomposition of all inorganic salt residues.
- If your primary focus is Catalytic Performance: Prioritize the precision of the temperature stability to achieve high crystallinity without causing structural collapse or loss of surface area.
The Muffle Furnace is not merely a heater; it is the instrument that defines the final crystalline identity and functional capability of your nanomaterials.
Summary Table:
| Process Stage | Technical Function of Muffle Furnace | Material Outcome |
|---|---|---|
| Thermal Decomposition | High-temperature heating of precursors | Removal of volatile impurities and inorganic salts |
| Phase Transformation | Sustained energy input for atomic rearrangement | Conversion from precursor to stable metal oxide |
| Annealing (600 °C) | Controlled environment for crystallization | Formation of the specific CoMn2O4 nano-spinel lattice |
| Microstructure Tuning | Uniform thermal history and stability | Optimized surface area and porosity for catalysis |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision is the difference between a successful catalyst and a failed experiment. KINTEK provides industry-leading high-temperature solutions—including Muffle, Tube, Rotary, and Vacuum furnaces—specifically designed to meet the rigorous demands of nano-spinel synthesis and calcination.
Backed by expert R&D and precision manufacturing, our systems offer the temperature stability and uniformity required to achieve high crystallinity without compromising material microstructure. Whether you need a standard setup or a customizable system for unique research needs, KINTEK is your partner in lab excellence.
Ready to optimize your material performance? Contact our technical experts today and find the perfect furnace for your laboratory.
Visual Guide
References
- T. C. Zhao, Xiaogang Wu. Heterogeneous Activation of NaClO by Nano-CoMn2O4 Spinel for Methylene Blue Decolorization. DOI: 10.3390/ijms26030940
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What software features should be considered when selecting a muffle furnace? Optimize Your Thermal Processes with Precision
- What are the common applications of muffle furnaces? Essential Uses in Labs and Industry
- What are some key features of premium muffle furnaces? Unlock Superior Performance and Safety
- What are some important 'Do's' when operating a muffle furnace? Ensure Safety and Efficiency in Your Lab
- What potential hazards are associated with benchtop furnaces? Essential Safety Guide for Lab Users
- How does a high-temperature box resistance furnace contribute to TWIP steel homogenization? Master Chemical Uniformity
- How should metal materials with grease be handled in a muffle furnace? Prevent Damage and Extend Furnace Life
- What are the differences between standard and high-temperature muffle furnaces? Choose the Right Furnace for Your Lab Needs



















