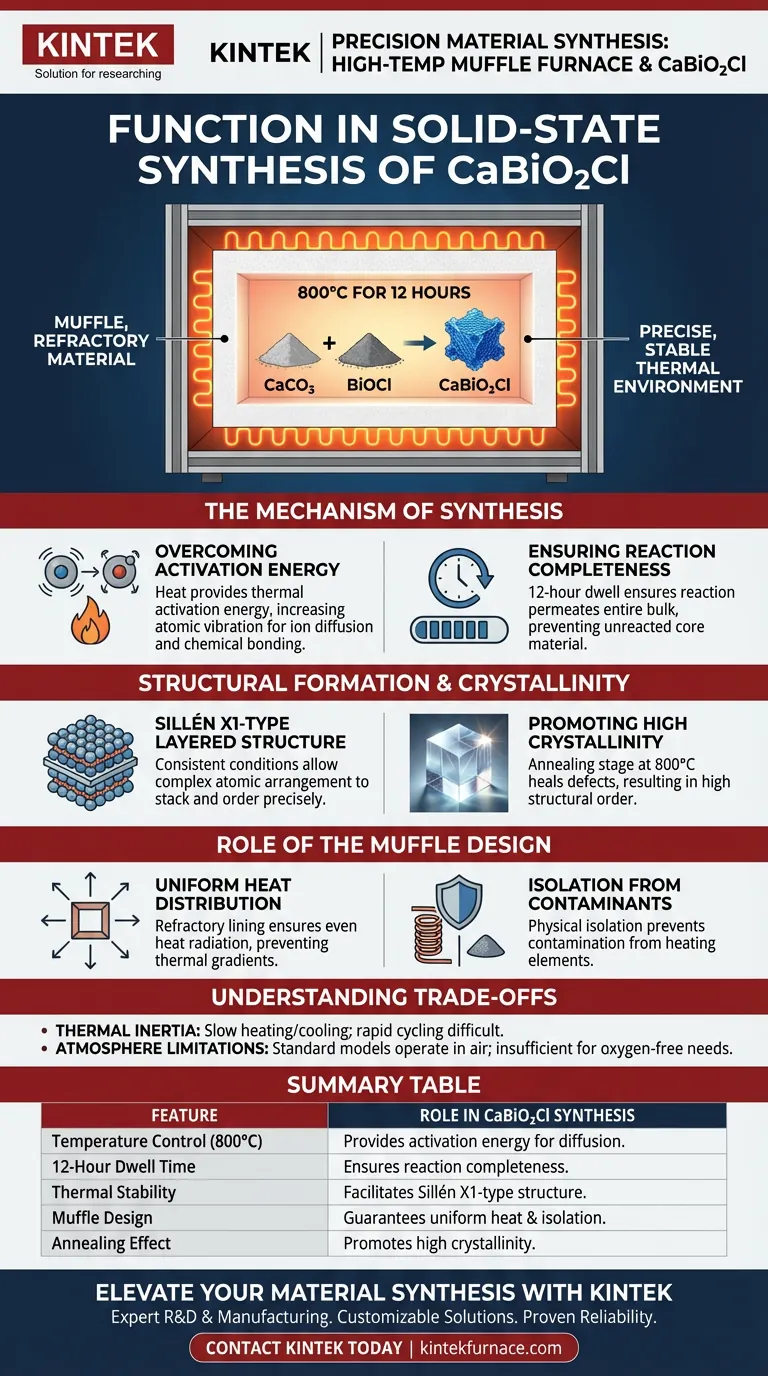
The primary function of a high-temperature muffle furnace in this context is to provide a precise, stable thermal environment. specifically maintained at 800°C for a duration of 12 hours. This sustained heat is the catalyst that forces the raw materials—Calcium Carbonate (CaCO3) and Bismuth Oxychloride (BiOCl)—to overcome their solid-state inertia and react chemically to form Calcium Bismuth Oxychloride (CaBiO2Cl).
By maintaining strict thermal stability, the furnace ensures the complete conversion of reactants into a specific Sillén X1-type layered structure, characterized by high crystallinity.

The Mechanism of Synthesis
Overcoming Activation Energy
Solid-state reactions are inherently sluggish compared to liquid or gas reactions because the atoms are locked in place.
The muffle furnace provides the necessary thermal activation energy.
By heating the mixture to 800°C, the furnace increases atomic vibration, allowing ions to diffuse across the boundaries of the solid particles and bond chemically.
Ensuring Reaction Completeness
The duration of the heating process is as critical as the temperature.
The furnace maintains this environment for 12 hours to ensure the reaction permeates the entire bulk of the material.
If the time were shortened, unreacted CaCO3 or BiOCl might remain in the core of the sample, compromising the purity of the final product.
Structural Formation and Crystallinity
Developing the Sillén X1-Type Structure
The specific target of this synthesis is a Sillén X1-type layered structure.
This complex atomic arrangement requires a slow, steady supply of energy to form correctly.
The muffle furnace provides the consistent thermodynamic conditions required for these layers to stack and order themselves precisely.
Promoting High Crystallinity
Crystallinity refers to the degree of structural order in a solid.
The stable 800°C environment acts as an annealing stage, allowing defects in the crystal lattice to heal.
This results in a final product with high crystallinity, which is often essential for the material's specific optical or electronic properties.
The Role of the "Muffle" Design
Uniform Heat Distribution
Unlike direct flame heating, a muffle furnace utilizes a refractory lining (the "muffle") to separate the sample from the heating elements.
This design feature ensures that the heat radiates evenly from all sides.
Uniform heating prevents thermal gradients within the sample, which could otherwise lead to uneven reaction rates or structural cracking.
Isolation from Contaminants
The muffle design physically isolates the CaBiO2Cl precursors from direct contact with the heating coils.
This prevents contamination from the heating element materials (such as resistance wire scale).
It ensures that the chemical composition of the final product remains stoichiometrically accurate to the initial mixture.
Understanding the Trade-offs
Thermal Inertia
Muffle furnaces generally have high thermal mass, meaning they heat up and cool down slowly.
While this is excellent for stability at 800°C, it makes rapid temperature cycling difficult.
You cannot easily quench the sample (cool it instantly) inside the furnace; the process requires a controlled cooling phase.
Atmosphere Limitations
While some muffle furnaces allow for gas flow, standard models operate in air.
If the synthesis of CaBiO2Cl required a strictly oxygen-free environment (to prevent oxidation of a specific dopant, for example), a standard muffle furnace might be insufficient compared to a tube furnace with vacuum capabilities.
However, for this specific reaction involving oxides and chlorides, the standard oxidative environment is generally acceptable.
Making the Right Choice for Your Goal
To ensure the successful synthesis of CaBiO2Cl, apply the following principles based on your priorities:
- If your primary focus is Phase Purity: Verify the furnace's temperature calibration strictly; even a slight deviation from 800°C can result in incomplete reactions or unwanted secondary phases.
- If your primary focus is Structural Perfection: Do not rush the 12-hour dwell time; the high crystallinity of the Sillén X1 structure depends on this prolonged exposure to organize the lattice.
Ultimately, the muffle furnace is not just a heater; it is the precision instrument that dictates the structural integrity of your final material.
Summary Table:
| Feature | Role in CaBiO2Cl Synthesis |
|---|---|
| Temperature Control (800°C) | Provides activation energy for ion diffusion and solid-state reaction. |
| 12-Hour Dwell Time | Ensures reaction completeness and eliminates unreacted precursors. |
| Thermal Stability | Facilitates the formation of the specific Sillén X1-type layered structure. |
| Muffle Design | Guarantees uniform heat distribution and isolation from contaminants. |
| Annealing Effect | Promotes high crystallinity by allowing crystal lattice defects to heal. |
Elevate Your Material Synthesis with KINTEK
Precise thermal environments are the difference between a successful synthesis and a failed experiment. KINTEK provides industry-leading muffle, tube, and vacuum furnaces designed to deliver the exact stability required for complex reactions like CaBiO2Cl production.
Why choose KINTEK?
- Expert R&D & Manufacturing: Our systems are engineered for high-precision temperature uniformity.
- Customizable Solutions: Whether you need a standard muffle furnace or a specialized CVD system, we tailor our equipment to your unique lab requirements.
- Proven Reliability: Trusted by researchers to achieve high crystallinity and phase purity every time.
Contact KINTEK today to find the perfect high-temperature solution for your synthesis needs!
Visual Guide
References
- Yu‐Yun Lin, Chiing‐Chang Chen. Visible-Light-Driven Photocatalysis of Carbon Dioxide and Organic Pollutants by CaBiO2Cl/g-C3N4. DOI: 10.3390/molecules30183760
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is a box furnace? A Versatile Tool for Precise Heat Treatment
- How does a muffle furnace contribute to kaolin-modified biochar? Optimize Pyrolysis & Mineral Integration
- What role does a high-temperature muffle furnace play in the green synthesis of TiO2? Key Phases for Pure Nanoparticles
- What are the potential disadvantages of muffle furnaces? Key Trade-offs for Lab Precision
- What is the primary purpose of using a muffle furnace for Bi5O7NO3 synthesis? Master Phase & Thermal Transformation
- Why is a laboratory high-temperature oven necessary for heterogeneous decatungstate catalysts? Ensure Structural Fixation
- What role does an industrial High-temperature Electric Furnace play? Achieve Precise Carbon Steel Standardization
- What functions does the calcination process in an industrial high-temperature muffle furnace perform? Catalyst Prep Guide



















