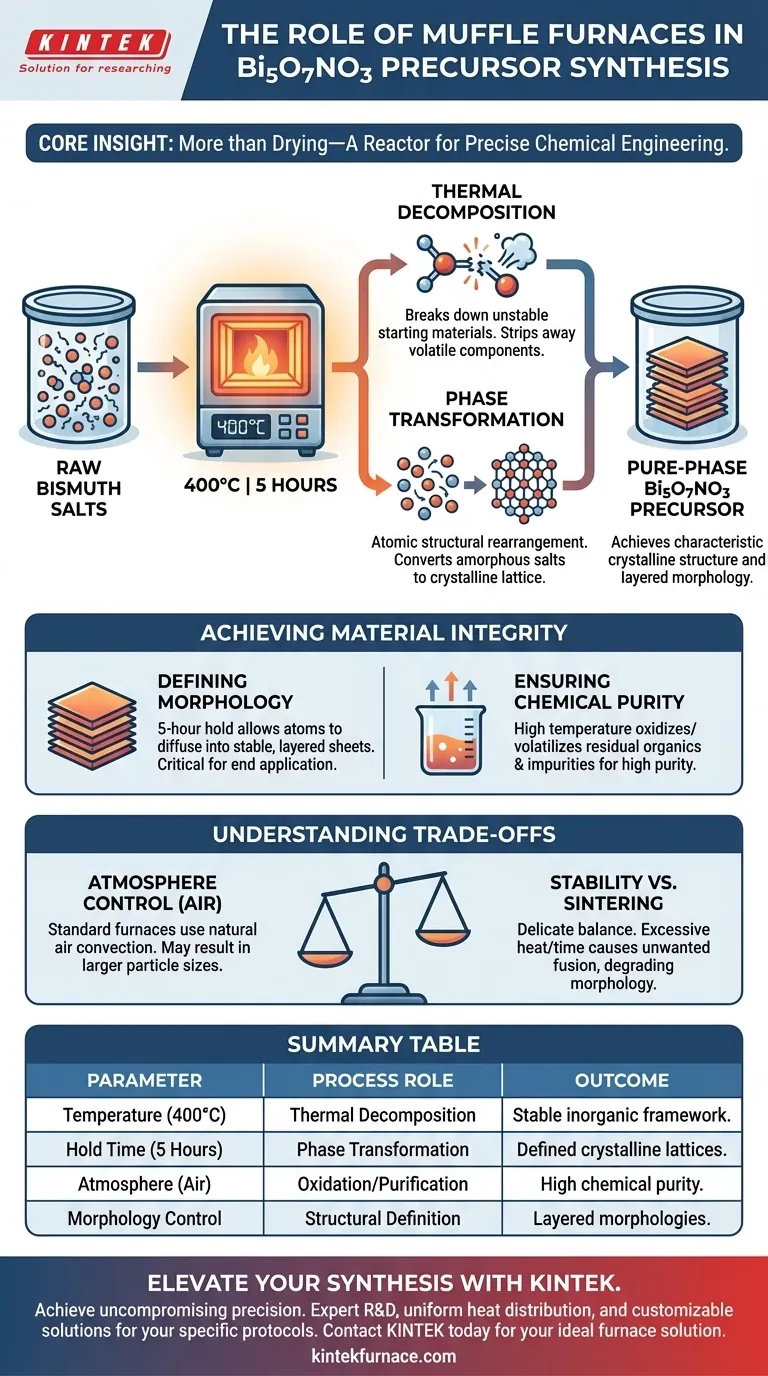
The primary purpose of using a muffle furnace in this synthesis is to facilitate thermal decomposition and phase transformation. By maintaining a controlled temperature of 400°C for 5 hours, the furnace converts raw bismuth salts into pure-phase Bi5O7NO3 precursors. This specific thermal treatment is essential for developing the material's characteristic crystalline structure and layered morphology.
Core Insight: The muffle furnace does not simply dry the material; it acts as a reactor for precise chemical engineering. It drives the energetic transition from raw chemical salts into a stable, structured inorganic framework through controlled high-temperature exposure.

The Mechanism of Transformation
Thermal Decomposition
The fundamental function of the muffle furnace in this context is to break down the starting materials. At 400°C, the bismuth salt raw materials become unstable and undergo thermal decomposition.
This process strips away volatile components from the raw salts. It effectively isolates the desired bismuth and oxygen components needed for the final precursor.
Phase Transformation
Simultaneously, the heat induces a phase transformation. This is a structural rearrangement at the atomic level, converting the amorphous or disordered decomposed salts into a defined crystalline lattice.
This transformation is what creates the "pure phase" Bi5O7NO3. Without this specific heat treatment, the material would remain a mixture of raw salts rather than a unified chemical compound.
Achieving Material Integrity
Defining Morphology
The duration of the treatment—specifically the 5-hour hold—allows the material to settle into a stable physical shape. The primary reference notes that this process yields layered morphologies.
This structural definition is critical for the material's end application. The high temperature provides the energy required for atoms to diffuse and arrange themselves into these specific layered sheets.
Ensuring Chemical Purity
While the primary focus is on forming Bi5O7NO3, the calcination process also serves as a purification step. High-temperature environments generally facilitate the removal of residual organics or solvents used in earlier synthesis stages.
By subjecting the precursors to 400°C, any remaining impurities or unreacted starting materials are likely oxidized or volatilized. This results in a final powder that possesses the high purity required for advanced applications.
Understanding the Trade-offs
Atmosphere Control Limitations
Standard muffle furnaces typically operate in an air environment. While effective for basic oxidation and calcination, they lack the specific atmosphere control (such as pure oxygen) found in specialized atmosphere furnaces.
As noted in supplementary studies on other oxides, specific atmospheres can inhibit volume diffusion and promote surface diffusion to reduce particle size. Using a standard muffle furnace means you rely on natural air convection, which may result in larger particle sizes compared to atmosphere-controlled processing.
Thermal Stability vs. Sintering
There is a delicate balance between achieving phase transformation and inducing unwanted sintering. While 400°C is necessary for formation, excessive heat or duration can cause particles to fuse.
This would degrade the desired layered morphology and reduce surface area. The specific protocol of 400°C for 5 hours is likely optimized to maximize crystallinity while minimizing agglomeration.
Making the Right Choice for Your Goal
To optimize the synthesis of Bi5O7NO3 precursors, consider your specific objectives:
- If your primary focus is Phase Purity: Adhere strictly to the 400°C temperature setpoint to ensure complete thermal decomposition of bismuth salts without melting the structure.
- If your primary focus is Structural Definition: Ensure the 5-hour duration is uninterrupted to allow sufficient time for the atomic rearrangement into layered morphologies.
- If your primary focus is Particle Size: Be aware that a standard air muffle furnace may result in larger particles than an atmosphere-controlled furnace; post-calcination grinding may be required.
Precision in thermal treatment is the single most critical factor in defining the chemical identity of your precursor.
Summary Table:
| Parameter | Process Role | Outcome for Bi5O7NO3 |
|---|---|---|
| Temperature (400°C) | Thermal Decomposition | Converts raw bismuth salts into a stable inorganic framework. |
| Hold Time (5 Hours) | Phase Transformation | Ensures complete atomic rearrangement into defined crystalline lattices. |
| Atmosphere (Air) | Oxidation/Purification | Removes volatile impurities and residual solvents for high chemical purity. |
| Morphology Control | Structural Definition | Facilitates the development of characteristic layered morphologies. |
Elevate Your Precursor Synthesis with KINTEK
Achieve uncompromising precision in your high-temperature calcination processes. Whether you are synthesizing Bi5O7NO3 precursors or advanced ceramics, KINTEK provides the specialized thermal equipment necessary for exact phase transformation and material integrity.
Why Choose KINTEK?
- Expert R&D & Manufacturing: Our systems are engineered for uniform heat distribution and stable temperature control.
- Versatile Solutions: From standard Muffle and Tube Furnaces to advanced Vacuum, CVD, and Rotary systems.
- Customizable for Your Needs: We tailor high-temp lab furnaces to meet your specific research or production protocols.
Ready to optimize your material morphology and purity? Contact KINTEK today to find your ideal furnace solution!
Visual Guide
References
- Jiaying Yan, Shunsuke Yagi. Defect‐Driven Reconstruction of Bismuth Nanoflowers via Precursor Engineering for Highly Efficient CO<sub>2</sub>‐to‐Formate Electrochemical Reduction. DOI: 10.1002/smsc.202500296
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What should be done if the silicon carbon rod in the muffle furnace's resistance furnace ages or underperforms? Expert Tips for Optimal Performance
- What function does a high-temperature box furnace serve in LLZO sintering? Achieve Superior LLZO Densification
- What role does temperature control play in a muffle furnace? Unlock Precision and Reliability for Your Lab
- How are box type resistance furnaces used in the manufacturing of electronic components? Essential for Precise Thermal Processing
- What is the purpose of an air-chamber laboratory furnace? Master Inorganic Glass and Ceramic Conversion
- What are the power requirements for the muffle furnace? Ensure Safe and Efficient Operation
- What are the applications of a laboratory muffle furnace in biochar evaluation? Optimize Your Biomass Research
- What are the potential disadvantages of muffle furnaces? Key Trade-offs for Lab Precision



















