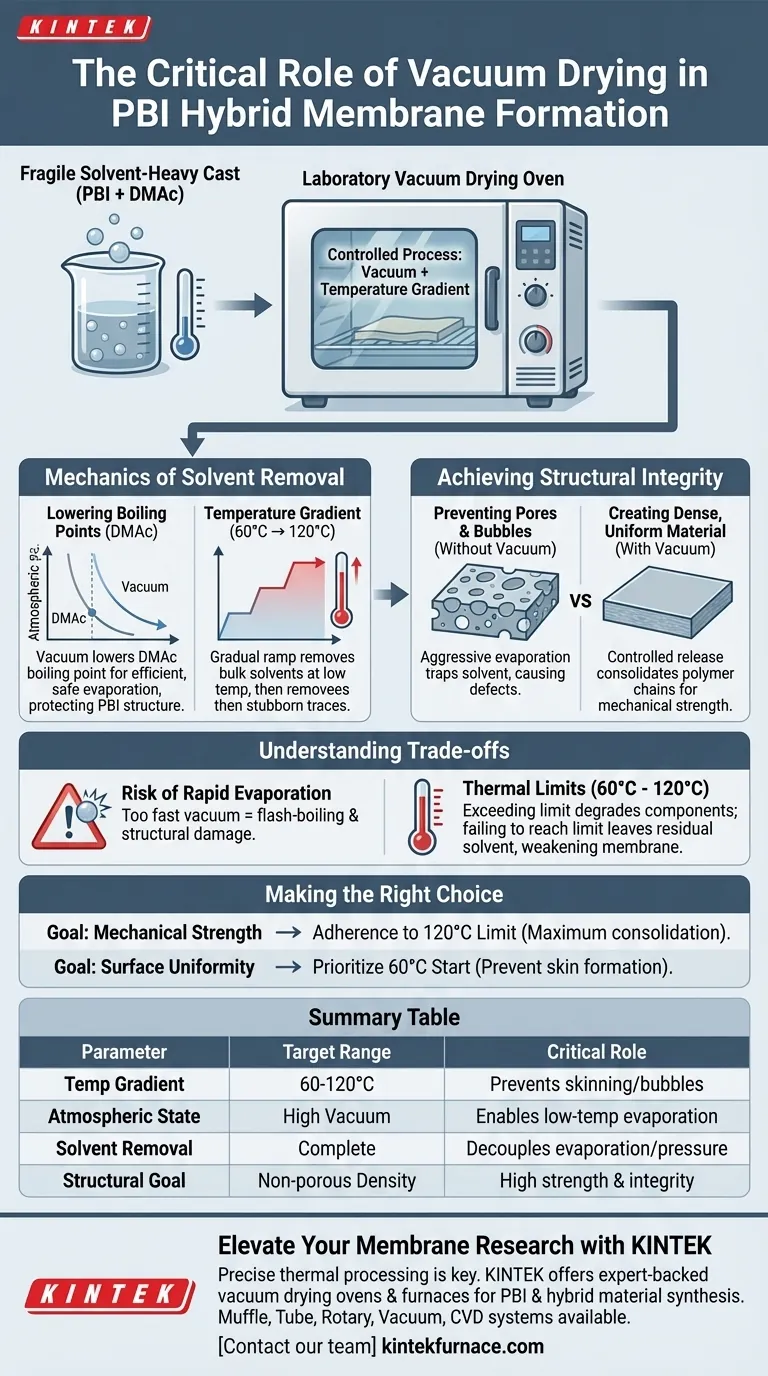
The laboratory vacuum drying oven is the critical processing tool used to solidify and densify Polybenzimidazole (PBI) hybrid membranes. Its primary function is the complete removal of residual solvents, specifically Dimethylacetamide (DMAc), using a controlled temperature gradient between 60°C and 120°C. By operating under vacuum, the oven lowers the boiling point of these solvents, allowing for accelerated evaporation without the formation of destructive bubbles or pores.
The vacuum drying process transforms a fragile, solvent-heavy cast into a dense, mechanically strong proton exchange membrane by decoupling temperature from evaporation pressure.

The Mechanics of Solvent Removal
Lowering Effective Boiling Points
PBI membranes are often cast using solvents like Dimethylacetamide (DMAc), which have high boiling points.
Removing these at atmospheric pressure would require excessive heat that could degrade the polymer.
The vacuum environment significantly reduces the boiling point of DMAc. This allows the solvent to evaporate efficiently at safer, lower temperatures, protecting the chemical structure of the PBI.
The Importance of the Temperature Gradient
You cannot simply blast the membrane with maximum heat immediately.
The process requires a stepped temperature gradient, specifically moving from 60°C up to 120°C.
This gradual ramp-up ensures that bulk solvents are removed first at lower temperatures, followed by the extraction of stubborn, bound traces as the temperature rises.
Achieving Structural Integrity
Preventing Pore and Bubble Formation
The most significant risk during membrane drying is the formation of voids.
If a solvent evaporates too aggressively or gets trapped under a dried surface skin, it creates bubbles and pores.
The vacuum oven specifically prevents this by ensuring a consistent, controlled release of solvent molecules from the entire thickness of the membrane.
Creating a Dense, Uniform Material
For a proton exchange membrane to function, it must be dense and non-porous.
The vacuum drying process consolidates the polymer chains as the solvent leaves.
This results in a uniform, compact structure that is mechanically strong and free of defects that would otherwise lead to failure during operation.
Understanding the Trade-offs
The Risk of Rapid Evaporation
While vacuum accelerates drying, applying too much vacuum too quickly can be counterproductive.
If the pressure drops too fast, the solvent may flash-boil rather than evaporate smoothly.
This rapid expansion can tear the microscopic structure of the membrane, creating the very surface defects you are trying to avoid.
Thermal Limits
Strict adherence to the 60°C to 120°C range is vital.
Exceeding the upper limit of this gradient before the solvent is fully removed can lock in stresses or degrade the hybrid components.
Conversely, failing to reach the upper 120°C threshold often leaves residual DMAc within the matrix, plasticizing the membrane and weakening its mechanical strength.
Making the Right Choice for Your Goal
To ensure the best results when processing PBI membranes, tailor your approach to your specific performance metrics:
- If your primary focus is Mechanical Strength: strict adherence to the upper 120°C limit is required to ensure complete solvent removal and maximum polymer chain consolidation.
- If your primary focus is Surface Uniformity: prioritize the lower end of the gradient (starting at 60°C) to prevent rapid skin formation that traps internal bubbles.
By precisely controlling the vacuum and thermal gradient, you ensure the membrane transitions from a chemical solution into a robust engineering material.
Summary Table:
| Process Parameter | Target Range | Critical Role in PBI Membrane Formation |
|---|---|---|
| Temperature Gradient | 60°C to 120°C | Gradual ramping prevents surface skinning and trapped bubbles. |
| Atmospheric State | High Vacuum | Lowers DMAc boiling point; enables low-temp evaporation without degradation. |
| Solvent Removal | Complete Extraction | Decouples evaporation from pressure to ensure maximum polymer consolidation. |
| Structural Goal | Non-porous Density | Prevents void formation to ensure high mechanical strength and integrity. |
Elevate Your Membrane Research with KINTEK
Precise thermal processing is the difference between a fragile cast and a high-performance proton exchange membrane. KINTEK provides industry-leading laboratory vacuum drying ovens and specialized high-temp furnaces designed to handle the rigorous demands of PBI and hybrid material synthesis.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique solvent extraction and density requirements.
Ready to optimize your material integrity? Contact our technical team today to find the perfect drying solution for your lab.
Visual Guide
References
- Ryo Kato, Atsunori Matsuda. Phosphoric Acid‐Immobilized Polybenzimidazole Hybrid Membranes with TiO<sub>2</sub> Nanowires for High‐Temperature Polymer Electrolyte Membrane Fuel Cells. DOI: 10.1002/celc.202500238
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What is the primary purpose of using nano-magnesium oxide as a template? Optimize Sulfur-Doped Porous Carbon Synthesis
- Why do Axial Flame Burners produce high NOx? Managing Thermal Intensity in Oxygen-Enhanced Combustion
- Why a 1:4 KOH Ratio and 1000 °C are Essential for Chemical Activation? Achieving Ultra-High Surface Area
- How does the precise control of heating rates affect sewage sludge biochar? Master Stability & Metal Stabilization
- What is the purpose of using an Argon Stream during activation? Enhance $CO_2$ Adsorption Efficiency
- Why is a specialized roasting simulation device necessary? Optimize Iron Ore Pellet Quality and Strength
- Why is a constant temperature drying oven required for processing lignin residue? Ensure Superior Pore Dehydration
- What is the function of a Teflon-lined autoclave in hydrothermal acid treatment? Enhance Catalyst Synthesis Efficiency



















