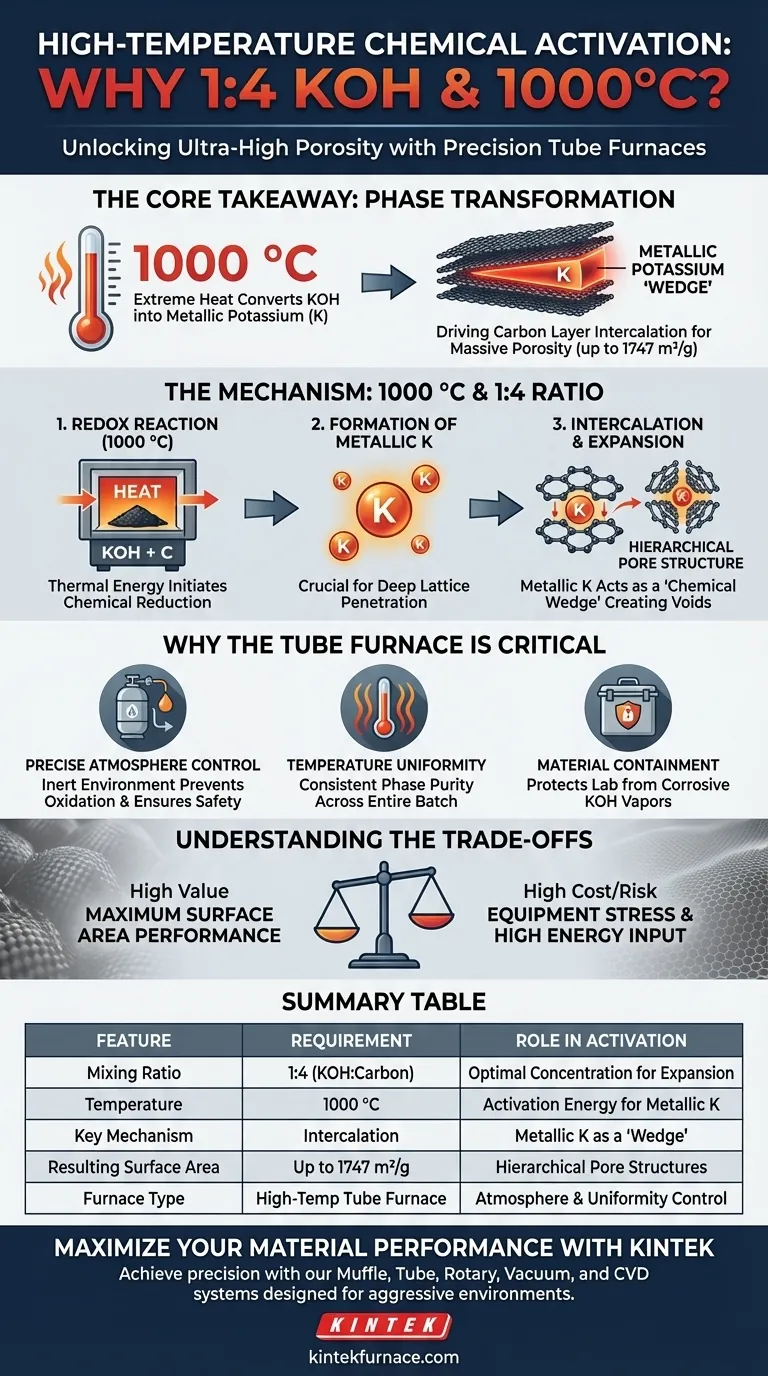
The combination of a 1:4 mixing ratio of KOH and a 1000 °C environment is driven by the need to induce a specific, aggressive chemical transformation that cannot occur at lower temperatures or concentrations.
At 1000 °C, the environment triggers a powerful redox reaction where the KOH is chemically reduced to metallic potassium. This metallic potassium forcibly intercalates (inserts itself) between the carbon layers of your material, physically expanding the lattice. This extreme process is the primary mechanism for generating hierarchical pore structures and achieving ultra-high specific surface areas (up to 1747 m²/g).
Core Takeaway The necessity of these extreme conditions lies in phase transformation: the 1000 °C heat provides the activation energy to convert KOH into metallic potassium. This metallic agent acts as a "chemical wedge," driving apart carbon layers to create the massive porosity required for high-performance ion adsorption.

The Mechanism of High-Temperature Activation
Driving the Redox Reaction
The 1000 °C set point is not arbitrary; it provides the thermal activation energy required to initiate a strong redox reaction between the carbonized material and the KOH.
Below this temperature threshold, the reaction may remain incomplete or superficial. The high thermal energy ensures the chemical kinetics are fast enough to fully process the material within the furnace's heating zone.
Formation of Metallic Potassium
The critical chemical event defined by these conditions is the reduction of Potassium Hydroxide (KOH) into metallic potassium.
This phase change is essential because ionic KOH reacts differently than metallic potassium. It is the metallic form that possesses the unique ability to penetrate deep into the carbon lattice structure.
Intercalation and Pore Expansion
Once generated, the metallic potassium intercalates into the carbon layers.
Imagine this process as inflating a balloon inside a stack of paper. The potassium forces the carbon layers apart, causing significant lattice expansion. When the potassium is later washed away, it leaves behind a complex network of voids, resulting in a hierarchical pore structure.
Why the Tube Furnace is Critical
Precise Atmosphere Control
The supplementary data notes that tube furnaces offer adjustable atmospheres (vacuum, reducing, or inert gases).
This is vital when working with metallic potassium at 1000 °C. The furnace allows you to maintain a strictly controlled environment (likely inert) to prevent the metallic potassium from oxidizing prematurely or reacting explosively with uncontrolled air, ensuring the safety and chemical purity of the process.
Temperature Uniformity
Achieving a consistent pore structure requires that every gram of the mixture experiences the exact same temperature.
High-temperature tube furnaces utilize advanced PID controllers and specific heating elements (like SiC or MoSi2) to ensure high temperature uniformity. This guarantees that the phase purity and crystal structure modifications are consistent throughout the entire sample batch.
Material Containment
Heating corrosive alkalis like KOH to 1000 °C presents significant containment challenges.
Tube furnaces are designed with specific tube materials (such as alumina or specialized alloys) and secure door mechanisms. This isolation protects the lab environment and the heating elements from the corrosive vapors generated during the activation process.
Understanding the Trade-offs
Equipment Stress and Corrosion
While necessary for activation, 1000 °C is an aggressive environment for furnace components.
KOH vapors are highly corrosive to many ceramics and heating elements. Even with a robust tube furnace, the lifespan of the tube (quartz, alumina, or alloy) may be reduced due to the severity of the chemical attack required to achieve high surface area.
Energy and Efficiency
Reaching and maintaining 1000 °C requires significant energy input.
While the furnace is designed for efficiency with rapid heating cycles, the process is inherently energy-intensive. You are trading energy efficiency for maximum surface area performance.
Making the Right Choice for Your Goal
When configuring your activation protocol, consider your specific end-goal requirements:
- If your primary focus is Maximum Surface Area: Stick to the 1000 °C protocol to ensure full conversion to metallic potassium and maximum lattice expansion (up to 1747 m²/g).
- If your primary focus is Process Safety and Equipment Longevity: Ensure your tube material is chemically resistant to alkali vapors at high temperatures and verify your gas purge capabilities are fully functional.
- If your primary focus is Reproducibility: Rely on the tube furnace’s PID controller to maintain strict uniformity, as even slight temperature drops will inhibit the formation of the metallic potassium "wedge."
Ultimately, the 1000 °C environment is the energetic price you pay to turn KOH into the metallic potassium tool needed to physically carve out ultra-high porosity.
Summary Table:
| Feature | Requirement | Role in Activation |
|---|---|---|
| Mixing Ratio | 1:4 (KOH:Carbon) | Provides optimal chemical concentration for lattice expansion |
| Temperature | 1000 °C | Provides activation energy to reduce KOH to metallic potassium |
| Key Mechanism | Intercalation | Metallic potassium acts as a 'wedge' to expand carbon layers |
| Resulting Surface Area | Up to 1747 m²/g | Creates hierarchical pore structures for ion adsorption |
| Furnace Type | High-Temp Tube Furnace | Ensures inert atmosphere control and thermal uniformity |
Maximize Your Material Performance with KINTEK
Achieve precision in every activation cycle with KINTEK’s industry-leading high-temperature solutions. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems designed to withstand the most aggressive chemical environments, including KOH activation. Whether you need customizable furnace designs or superior temperature uniformity for your lab research, KINTEK delivers the durability and control you require.
Ready to scale your material synthesis? Contact our experts today to find the perfect customizable furnace for your unique needs!
Visual Guide
References
- Dipendu Saha, David Young. Nanoporous Carbons from Hydrothermally Treated Alga: Role in Batch and Continuous Capacitive Deionization (CDI). DOI: 10.3390/molecules30132848
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- How does a high-precision reaction system aid methane CLR research? Unlock Advanced Syngas Insights
- How does an annealing furnace work? A Guide to Controlled Heat Treatment
- Why is precise temperature control necessary in high-temp furnaces for VN alloys? Master the Thermal Phase Switch
- How does magnetron sputtering equipment facilitate BSnO thin films? Precision Control for Semiconductor Bandgap Tuning
- How does high-temperature calcination affect kaolin? Boost Surface Area and Catalytic Reactivity via Thermal Processing
- Why must the entire system be maintained at a high temperature during the filling process of a sodium heat pipe?
- What are the benefits of ESR for carbonitride distribution in H13 steel? Enhance Your Material's Isotropic Properties
- What role does a laboratory circulating air drying oven play in the post-treatment of composite membranes? Master Stability



















