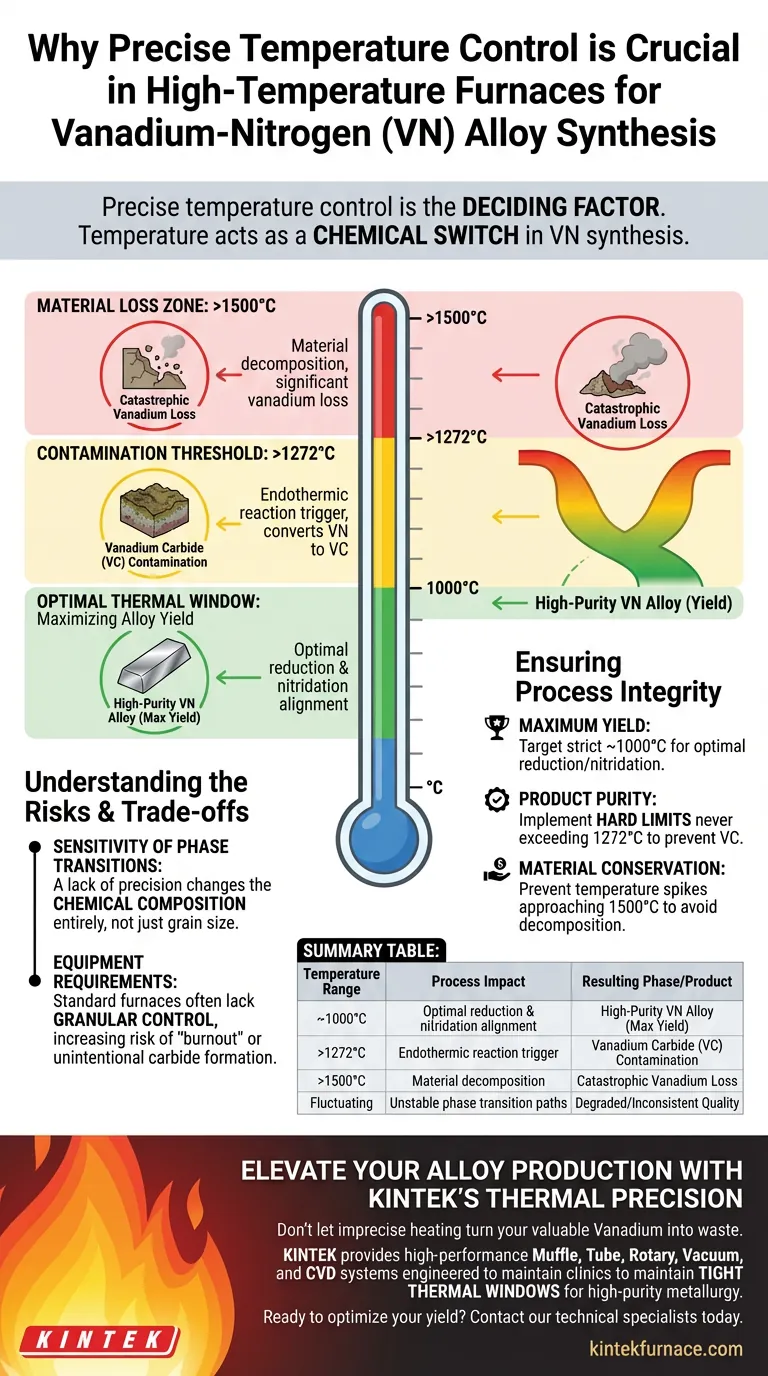
Precise temperature control is the deciding factor in the synthesis of Vanadium-Nitrogen (VN) alloys, dictating whether you produce a high-purity alloy or a degraded waste product. Because the process involves sequential reduction and nitridation reactions, the temperature serves as the primary variable that selects the specific phase transition path.
Temperature acts as a chemical switch in VN synthesis. Deviating from the optimal thermal window does not merely slow the process; it fundamentally alters the chemical reaction, converting valuable alloys into unwanted carbides or causing total material loss.

Defining the Critical Thermal Windows
The synthesis of VN is not a linear process where "hotter is better." It relies on adhering to specific thermal boundaries to maximize yield and prevent reverse reactions.
Maximizing Alloy Yield
Research identifies approximately 1000°C as the optimal temperature for VN synthesis.
At this specific thermal point, the reduction and nitridation reactions align to produce the highest yield of the desired Vanadium-Nitrogen phase. Maintaining this temperature is essential for efficient production.
The Threshold for Contamination
Precision is required to avoid crossing the 1272°C threshold.
Once the furnace temperature exceeds this limit, an endothermic reaction is triggered. This reaction chemically converts the desired VN into Vanadium Carbide (VC), essentially corrupting the purity of the final product.
Preventing Material Loss
Extreme overheating, specifically reaching 1500°C, leads to catastrophic process failure.
At this temperature, the material decomposes, leading to significant vanadium loss. This not only destroys the product but also represents a financial loss due to the waste of raw materials.
Understanding the Risks and Trade-offs
While high-temperature furnaces are capable of extreme heat, the "trade-off" in VN synthesis is that power must be sacrificed for precision.
The Sensitivity of Phase Transitions
The primary pitfall in this process is assuming that temperature fluctuations are harmless.
In many metallurgical processes, a small overshoot only affects grain size. In VN synthesis, a lack of precision changes the chemical composition entirely.
Equipment Requirements
Standard industrial furnaces often lack the granular control required to stay within the safe window (below 1272°C) while maintaining the 1000°C target.
Using equipment with low thermal precision increases the risk of "burnout" or unintentional carbide formation, even if the average temperature seems correct.
Ensuring Process Integrity
To maximize the quality of Vanadium-Nitrogen alloys, you must align your thermal strategy with the chemical realities of the material.
- If your primary focus is Maximum Yield: Target a strict holding temperature of approximately 1000°C to optimize the reduction and nitridation sequence.
- If your primary focus is Product Purity: Implement hard limits to ensure the internal furnace temperature never exceeds 1272°C to prevent the formation of Vanadium Carbide.
- If your primary focus is Material Conservation: Ensure your control loop prevents temperature spikes approaching 1500°C to avoid decomposition and vanadium loss.
Success in VN synthesis is not about generating heat, but about maintaining the discipline to stay within the boundaries where chemistry favors the alloy.
Summary Table:
| Temperature Range | Process Impact | Resulting Phase/Product |
|---|---|---|
| ~1000°C | Optimal reduction & nitridation alignment | High-Purity VN Alloy (Max Yield) |
| >1272°C | Endothermic reaction trigger | Vanadium Carbide (VC) Contamination |
| >1500°C | Material decomposition | Catastrophic Vanadium Loss |
| Fluctuating | Unstable phase transition paths | Degraded/Inconsistent Quality |
Elevate Your Alloy Production with KINTEK's Thermal Precision
Don't let imprecise heating turn your valuable Vanadium into waste. At KINTEK, we understand that in VN synthesis, temperature is the ultimate chemical switch. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems specifically engineered to maintain the tight thermal windows required for high-purity metallurgy.
Whether you need granular control to avoid carbide formation or customizable lab high-temp furnaces for unique material R&D, KINTEK delivers the accuracy your process demands.
Ready to optimize your yield? Contact our technical specialists today to find the perfect customizable furnace solution for your synthesis needs.
Visual Guide
References
- Xiaojie Cui, Yuekai Xue. Thermodynamic Study of Production of Vanadium–Nitrogen Alloy and Carbon Monoxide by Reduction and Nitriding of Vanadium Oxide. DOI: 10.3390/pr12091839
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What are the equipment requirements for o-LISO ceramic sintering? Achieve 1050°C Precision for High Conductivity
- Why is a forced convection drying oven required for concrete moisture experiments? Achieve Precise Baseline Accuracy
- What is the role of industrial thermometers in monitoring thermal stress? Ensure Safety via High-Precision Data
- How does a symmetric suction design improve steel wire heat treatment? Achieve Uniform Salt Flow and Sorbite Quality
- What is the technical purpose of the ball milling process for Ti12%Zr? Master Mechanical Activation & Alloying
- How does the thermal treatment enhance the mechanical properties of AZO and ZnO coatings? Boost Durability & Hardness
- What is the catalytic mechanism of methane gas conversion in Ni-Co CNT synthesis? Master Carbon Transformation
- How does a single-action hydraulic press ensure the quality of green compacts? Key Factors for Aluminum Composites



















