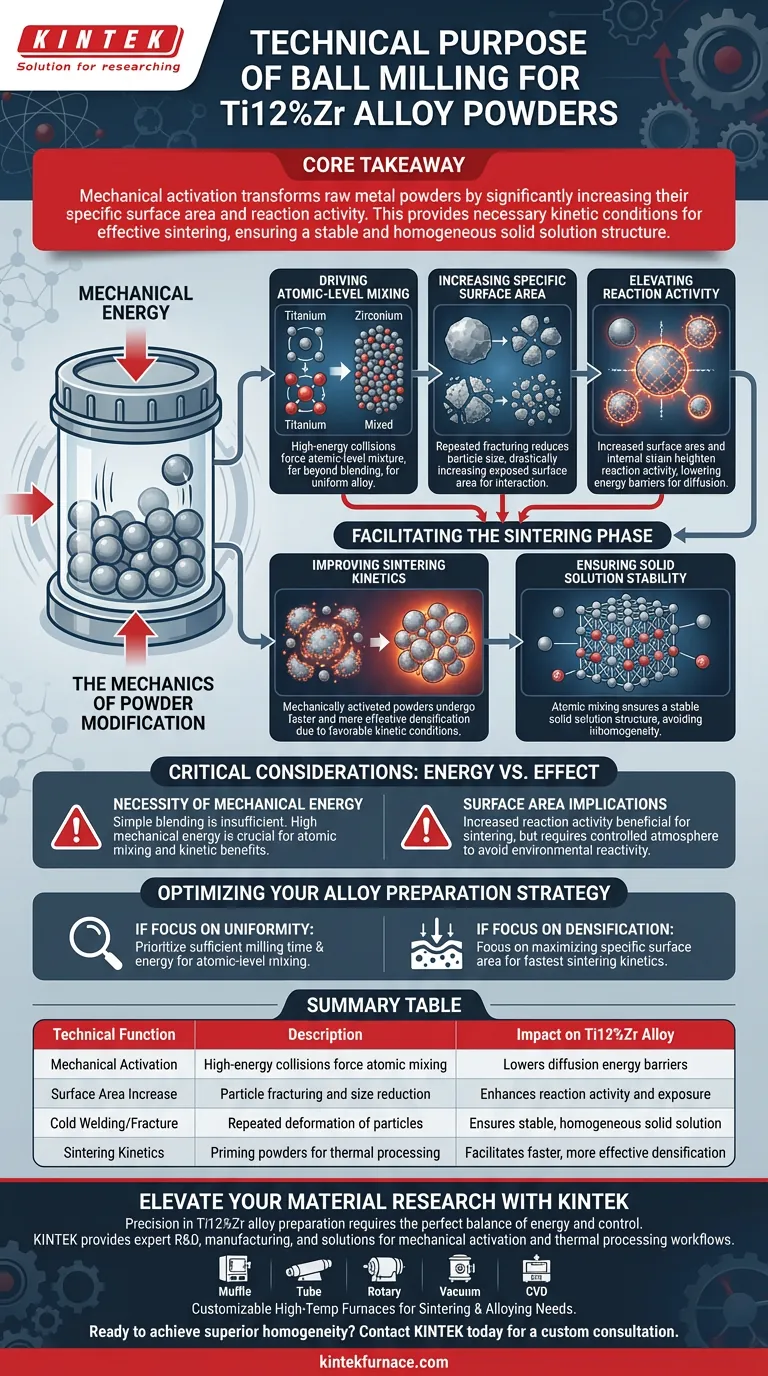
The primary technical purpose of ball milling in this context is mechanical activation. It utilizes high-energy collisions to force Titanium and Zirconium powders into an atomic-level mixture, going far beyond simple blending. This process fundamentally alters the physical state of the reactants to prepare them for successful alloying.
Core Takeaway Ball milling transforms raw metal powders by significantly increasing their specific surface area and reaction activity. This mechanical activation provides the necessary kinetic conditions for effective sintering, ensuring the final Ti12%Zr alloy achieves a stable and homogeneous solid solution structure.

The Mechanics of Powder Modification
Driving Atomic-Level Mixing
The ball milling process applies intense mechanical energy to the powder mixture.
This energy is not just for distribution; it forces the Titanium and Zirconium particles to interact at an atomic level. This intimate contact is the foundational step required to create a uniform alloy rather than a segregated mixture of two distinct metals.
Increasing Specific Surface Area
Through repeated fracturing and cold welding, the milling process reduces particle size and creates new surfaces.
This results in a drastic increase in the specific surface area of the powder. A larger surface area means more material is exposed and available for chemical and physical interaction during subsequent processing steps.
Elevating Reaction Activity
The combination of increased surface area and internal lattice strain introduced by milling heightens the reaction activity of the powders.
High reaction activity is crucial because it lowers the energy barriers required for the metals to diffuse into one another. The powder is effectively "primed" to react.
Facilitating the Sintering Phase
Improving Sintering Kinetics
Sintering relies on heat and diffusion to densify the material, but heat alone is often inefficient for passive powders.
Ball milling creates favorable kinetic conditions for this process. Because the powders are mechanically activated, densification occurs more readily and effectively, leading to a higher quality final product.
Ensuring Solid Solution Stability
The ultimate goal of preparing Ti12%Zr is to achieve a single, unified phase where the zirconium is fully dissolved in the titanium lattice.
The atomic mixing achieved during milling ensures the formation of a stable solid solution structure. Without this pre-processing step, the final alloy risks inhomogeneity or phase separation.
Critical Considerations: Energy vs. Effect
The Necessity of Mechanical Energy
It is vital to recognize that simple physical blending is insufficient for this alloy system.
The process explicitly relies on mechanical energy to achieve the described benefits. If the milling energy is too low, the atomic-level mixing will not occur, and the kinetic advantages for sintering will be lost.
Surface Area Implications
While increasing surface area is the goal, it creates a highly reactive state.
This increased reaction activity is beneficial for sintering but requires careful handling to maintain purity. The process creates a potential for increased reactivity with the environment if not managed within the controlled milling atmosphere.
Optimizing Your Alloy Preparation Strategy
To ensure the highest quality Titanium-12%Zirconium alloy, align your processing parameters with your specific structural goals.
- If your primary focus is Uniformity: Prioritize sufficient milling time and energy to guarantee atomic-level mixing before any thermal processing begins.
- If your primary focus is Densification: Focus on maximizing the specific surface area to drive the fastest and most complete sintering kinetics.
The success of the Ti12%Zr alloy depends not just on the ingredients, but on using mechanical force to unlock their chemical potential.
Summary Table:
| Technical Function | Description | Impact on Ti12%Zr Alloy |
|---|---|---|
| Mechanical Activation | Uses high-energy collisions to force atomic mixing | Lowers energy barriers for diffusion |
| Surface Area Increase | Particle fracturing and reduction in size | Enhances reaction activity and exposure |
| Cold Welding/Fracture | Repeated deformation of powder particles | Ensures a stable, homogeneous solid solution |
| Sintering Kinetics | Priming powders for thermal processing | Facilitates faster and more effective densification |
Elevate Your Material Research with KINTEK
Precision in Ti12%Zr alloy preparation requires the perfect balance of energy and control. KINTEK provides industry-leading solutions backed by expert R&D and manufacturing to streamline your mechanical activation and thermal processing workflows.
Whether you require high-performance Muffle, Tube, Rotary, Vacuum, or CVD systems, our lab high-temp furnaces are fully customizable to meet your unique sintering and alloying needs.
Ready to achieve superior homogeneity in your advanced materials?
Contact KINTEK today for a custom consultation.
Visual Guide
References
- El‐Sayed M. Sherif. A comparative study on the corrosion of pure titanium and titanium–12%zirconium alloy after different exposure periods of time in sodium chloride solution. DOI: 10.1063/5.0192701
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
People Also Ask
- What is the function of a Mass Flow Controller (MFC)? Achieve Precise Ethanol Vapor Delivery for Graphene Synthesis
- How is SEM utilized to evaluate manganese phosphate catalysts after calcination? Verify Nanosheet Integrity.
- Why is immediate quenching required after CTS treatment of mesoporous carbon? Preserve Your Material’s Atomic Structure
- What is the function of a laboratory oven in activated carbon preparation? Ensure Superior Material Stability
- Why is a vacuum drying oven used for BC-FeOOH biochar? Protect Reactivity and Prevent Particle Aggregation
- What is the purpose of using a laboratory electric thermostatic blast drying oven in the pretreatment of sludge? Efficiency & Accuracy
- What type of furnaces are commonly used for sintering? Choose the Right Furnace for Your Process
- Why is a furnace with high-precision temperature control required for DPKB-S? Ensuring Material Synthesis Accuracy

