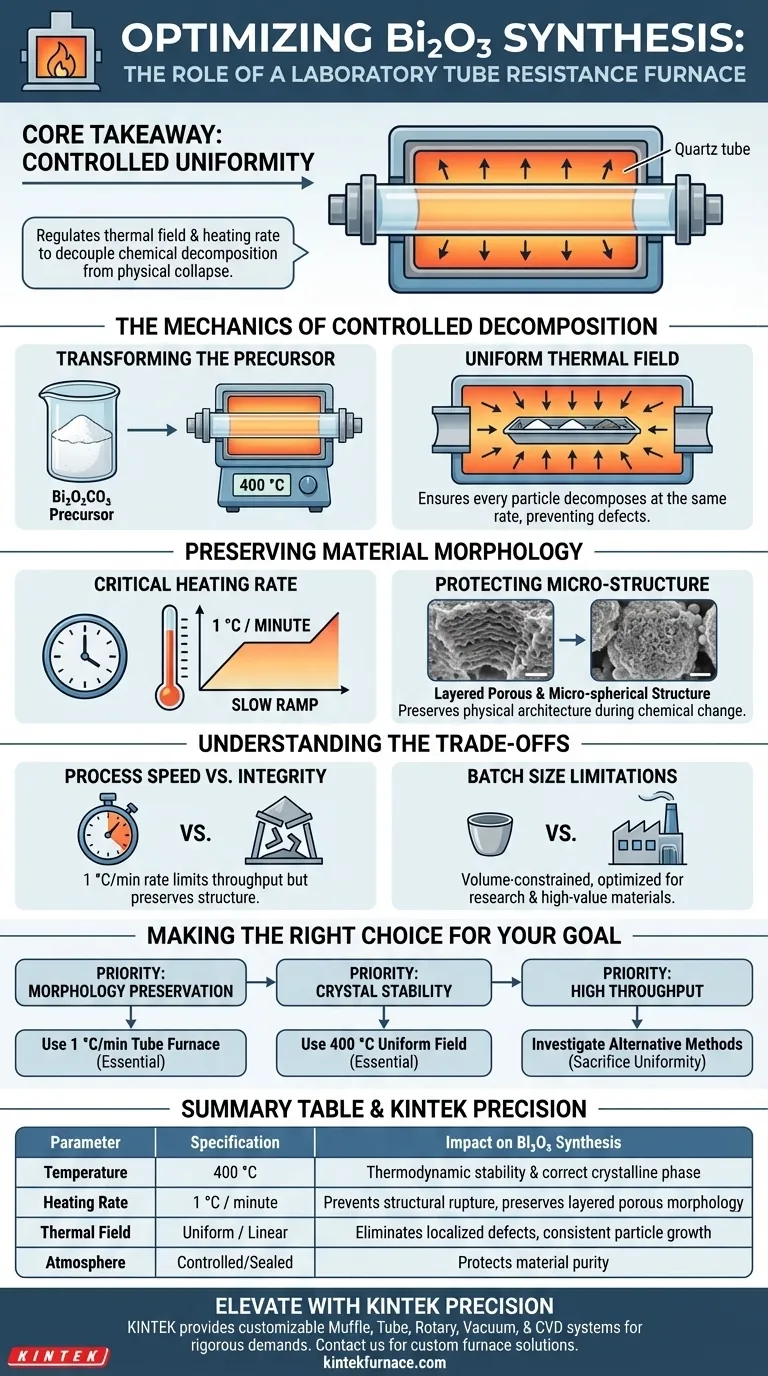
The laboratory tube resistance furnace serves as a precision instrument for the thermal decomposition of bismuth subcarbonate (Bi2O2CO3) precursors into stable bismuth oxide (Bi2O3). This process relies on a controlled 400 °C environment and a slow, specific heating rate of 1 °C per minute to ensure the transformation results in the correct crystalline phase without compromising the material's physical structure.
Core Takeaway The value of a tube furnace in this application is not merely high heat, but controlled uniformity. By regulating the thermal field and heating rate, the furnace decouples the chemical decomposition from physical collapse, allowing the synthesis of thermodynamically stable crystals that retain a complex, porous morphology.

The Mechanics of Controlled Decomposition
Transforming the Precursor
The primary function of the furnace in this context is to drive the thermal decomposition of Bi2O2CO3.
This precursor material is chemically converted into Bi2O3 crystals.
The furnace maintains a stable temperature of 400 °C, which is the thermodynamic sweet spot required to achieve a stable crystalline form of the oxide.
The Importance of a Uniform Thermal Field
Success depends on how evenly the heat is applied.
A tube resistance furnace creates a uniform thermal field around the sample.
This uniformity ensures that every particle within the batch undergoes decomposition at the exact same rate, preventing localized defects or inconsistent crystal growth.
Preserving Material Morphology
The Critical Heating Rate
The most distinct contribution of the tube furnace is its ability to execute a precise heating ramp.
For Bi2O3 precursors, the optimal rate is extremely slow: 1 °C per minute.
Rapid heating would likely cause the volatile components of the precursor to escape too violently, rupturing the material's structure.
Protecting the Micro-Structure
The slow ramp rate preserves the micro-spherical morphology of the particles.
It also protects the layered porous structure inherent to the precursor.
By controlling the heat input so precisely, the furnace allows the chemical structure to change while the physical architecture remains intact.
Understanding the Trade-offs
Process Speed vs. Structural Integrity
The strict requirement for a 1 °C/minute ramp rate creates a significant bottleneck in processing time.
While this slowness is essential for preserving the layered porous structure, it severely limits throughput compared to flash-heating methods.
Batch Size Limitations
Tube furnaces are inherently volume-constrained.
While they provide the sealed, controlled environment necessary for high-purity synthesis, they are generally not suitable for mass production.
They are optimized for research and high-value, low-volume material production where microstructure dictates performance.
Making the Right Choice for Your Goal
To determine if this specific thermal treatment setup aligns with your project requirements, consider the following:
- If your primary focus is Morphology Preservation: The 1 °C/min ramp rate in a tube furnace is non-negotiable to maintain porous, micro-spherical structures.
- If your primary focus is Crystal Stability: The uniform thermal field at 400 °C is essential for ensuring the complete thermodynamic stability of the Bi2O3 crystals.
- If your primary focus is High Throughput: You may need to investigate alternative heating methods, accepting that you will likely sacrifice the uniformity of the porous structure.
Precision in thermal treatment is the defining factor between a collapsed powder and a high-performance porous material.
Summary Table:
| Parameter | Specification | Impact on Bi2O3 Synthesis |
|---|---|---|
| Temperature | 400 °C | Ensures thermodynamic stability and correct crystalline phase. |
| Heating Rate | 1 °C / minute | Prevents structural rupture; preserves layered porous morphology. |
| Thermal Field | Uniform / Linear | Eliminates localized defects and ensures consistent particle growth. |
| Atmosphere | Controlled/Sealed | Protects material purity during chemical decomposition. |
Elevate Your Material Synthesis with KINTEK Precision
Precise morphology preservation in Bi2O3 precursors requires absolute control over thermal ramps and uniformity. KINTEK provides world-class laboratory solutions designed specifically for these rigorous demands.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique research or production needs. Whether you require slow-ramp stability or specialized atmospheres, our high-temp furnaces ensure your materials retain their complex architectures and performance characteristics.
Ready to optimize your thermal treatment? Contact KINTEK today to discuss your custom furnace requirements!
Visual Guide
References
- Fan Yang, Wanfeng Xie. Structural design of highly permeable Bi <sub>2</sub> O <sub>3</sub> microspheres decorated by Pt‐nanoparticles: facile synthesis and acetic acid sensing performance. DOI: 10.1007/s12598-025-03391-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What materials are commonly used for the heating element in tubular furnaces? Choose the Best for Your High-Temp Needs
- How does a high-temperature tube furnace facilitate the final ceramization of EG/LTO composites? Expert Insights
- What are the different types of tube furnaces available? Find the Perfect Fit for Your Lab's Needs
- What is the core role of a tube furnace in synthesizing magnetic carbon-based composites? Expert Insights
- What is the primary function of a Drop Tube Furnace (DTF)? Simulating Industrial Combustion for Research
- How vacuum pumping affects Zr2.5Nb nitriding? Achieve pure ZrN surfaces in high-temp tube furnaces.
- Why are the high-temperature carbonization and activation of sugarcane bagasse typically conducted in a tube furnace?
- How does a high-temperature tube furnace contribute to the pore regulation of carbon nanofibers? Precision Engineering



















