A laboratory tube furnace ensures the structural stability of cotton-derived hard carbon by maintaining a strictly controlled thermal and atmospheric environment during pyrolysis. By utilizing a high-precision temperature control system under a nitrogen protective atmosphere, the furnace manages the delicate transformation of cellulose molecules into a robust, amorphous carbon framework.
Core Takeaway The tube furnace guarantees quality by executing a precise heating protocol—typically a constant rate of 5 °C/min up to 1,000 °C—within an inert nitrogen environment. This prevents oxidative loss and facilitates the formation of an amorphous carbon structure with a consistent interlayer spacing of approximately 3.6-3.7 Å, which is the critical factor for the material's electrochemical stability.
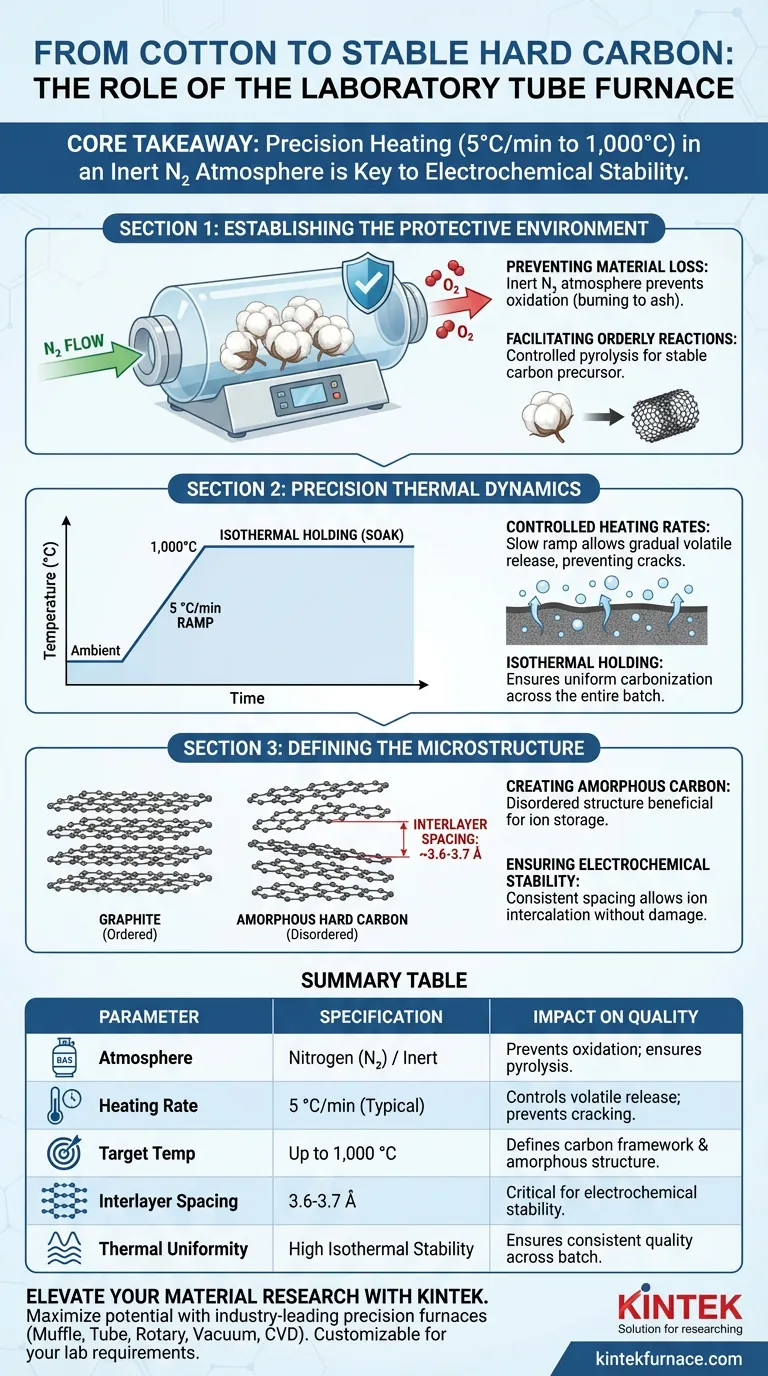
Establishing the Protective Environment
Preventing Material Loss
The primary threat to organic precursors like waste cotton at high temperatures is oxidation. If oxygen is present, the cotton will combust and turn to ash rather than carbonizing.
The Role of Inert Atmosphere
The tube furnace creates a sealed environment flushed with nitrogen gas. This inert atmosphere ensures that the cotton undergoes pyrolysis (thermal decomposition) rather than burning, preserving the carbon skeleton.
Facilitating Orderly Reactions
By eliminating reactive oxygen, the furnace forces the biomass to undergo orderly dehydrogenation and deoxygenation. This controlled chemical breakdown is necessary to leave behind a stable, carbon-rich precursor.
Precision Thermal Dynamics
Controlled Heating Rates
The structural integrity of hard carbon relies heavily on how fast the heat is applied. The furnace maintains a steady heating rate, such as 5 °C/min.
Managing Volatile Release
Waste cotton contains significant volatile components. A controlled ramp rate allows these volatiles to escape gradually rather than explosively, preventing cracks or structural collapse in the forming carbon matrix.
Isothermal Holding
Once the target temperature (e.g., 1,000 °C) is reached, the furnace provides stable isothermal holding. This "soak" period ensures the carbonization reaction penetrates the entire material volume uniformly.
Defining the Microstructure
Creating Amorphous Carbon
The thermal treatment transforms the cellulose into an amorphous carbon material. Unlike graphite, which has a highly ordered long-range structure, this material retains a disordered structure beneficial for specific storage applications.
Optimizing Interlayer Spacing
The specific thermal profile achieves a consistent interlayer spacing of approximately 3.6-3.7 Å. This spacing is wider than that of graphite, providing the necessary "room" for ions to intercalate without damaging the structure.
Ensuring Electrochemical Stability
The uniformity of this interlayer spacing dictates the final quality of the product. A consistent structure ensures that the hard carbon remains stable during repeated charge/discharge cycles in battery applications.
Understanding the Trade-offs
The Risk of Thermal Shock
If the heating rate is too aggressive (significantly faster than 5 °C/min), the rapid release of gases can destroy the pore structure. This results in a material with low mechanical strength and poor performance.
Temperature Precision vs. Energy Cost
Higher temperatures and longer holding times generally improve structural arrangement but increase energy consumption. There is a diminishing return where excessive heat might lead to unwanted graphitization, reducing the unique benefits of hard carbon.
Atmosphere Sensitivity
Even minor leaks in the nitrogen supply can introduce oxygen. This leads to surface oxidation, which creates defects that degrade the electrical conductivity and stability of the final hard carbon.
Making the Right Choice for Your Goal
To optimize your hard carbon synthesis, match your furnace settings to your specific material requirements:
- If your primary focus is Electrochemical Stability: Prioritize a slow, constant heating rate (5 °C/min) and a hold at 1,000 °C to ensure uniform interlayer spacing (3.6-3.7 Å).
- If your primary focus is Structural Integrity: Ensure the nitrogen flow is robust and continuous to prevent any oxidative etching of the carbon framework during pyrolysis.
- If your primary focus is Pore Structure Tuning: Consider using multi-stage heating profiles (e.g., pausing at lower temperatures) to control the rate of volatile release before the final carbonization.
The quality of your hard carbon is not defined by the raw cotton, but by the precision with which your furnace manages its thermal transformation.
Summary Table:
| Parameter | Specification/Requirement | Impact on Hard Carbon Quality |
|---|---|---|
| Atmosphere | Nitrogen (N2) / Inert | Prevents oxidation; ensures pyrolysis over combustion |
| Heating Rate | 5 °C/min (Typical) | Controls volatile release to prevent structural cracking |
| Target Temp | Up to 1,000 °C | Defines carbon framework and amorphous structure |
| Interlayer Spacing | 3.6-3.7 Å | Critical for electrochemical stability and ion storage |
| Thermal Uniformity | High Isothermal Stability | Ensures consistent material quality across entire batch |
Elevate Your Material Research with KINTEK
Maximize the potential of your biomass carbonization with industry-leading precision. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique lab requirements. Whether you are optimizing interlayer spacing for battery anodes or refining pore structures, our furnaces provide the stability your research demands.
Ready to achieve superior structural stability in your materials?
Contact KINTEK Today to Customize Your Furnace Solution
Visual Guide
References
- H. Sarma, Nolene Byrne. Effect of precursor morphology of cellulose-based hard carbon anodes for sodium-ion batteries. DOI: 10.3389/fbael.2023.1330448
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- How does a vacuum tube furnace work? Master Precise High-Temp Material Processing
- What are the pros and cons of vertical tube furnaces? Precision vs. Capacity for Your Lab
- Why is an industrial tube furnace required for the heat treatment of SiCN(Ni)/BN ceramics? Master Precise Pyrolysis
- What is the purpose of using a high-temperature tube furnace with a steam generator for LOCA simulation?
- Why is tube furnace temperature control critical for anhydrous rare earth halide powders? Achieve Precise Synthesis
- What is the advantage of a three-zone tube furnace? Achieve Larger, More Uniform Heating for Your Processes
- What role do high-performance box or tube furnaces play in LATP sintering? Master Densification & Ionic Conductivity
- What components are in a turn-key quartz tube furnace? Essential parts for precise atmospheric control.



















