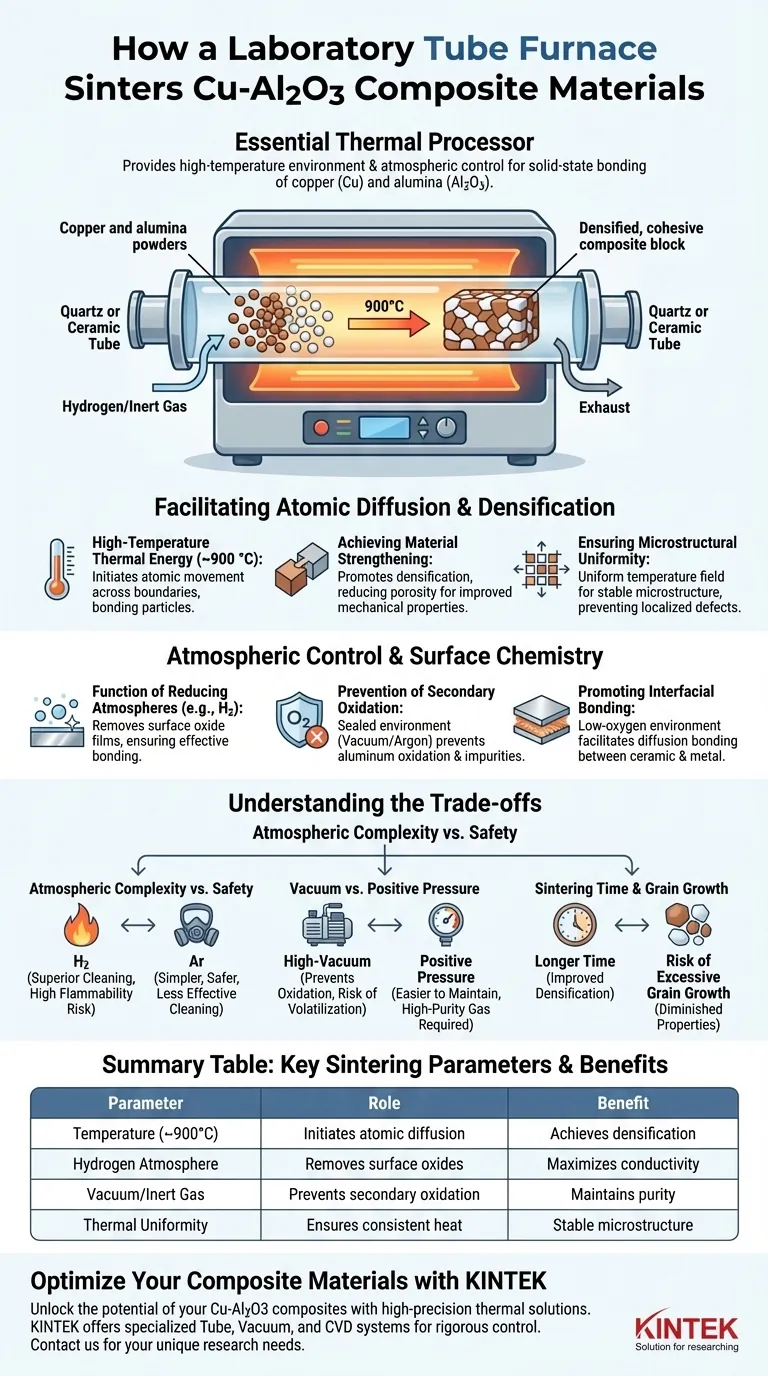
The laboratory tube furnace is the essential thermal processor for Cu-Al2O3 composites, providing the high-temperature environment and atmospheric control required for solid-state bonding. By operating at temperatures typically around 900 °C, the furnace provides the thermal energy necessary for atomic diffusion between copper and alumina particles. Simultaneously, it maintains a specialized chemical atmosphere—such as a hydrogen reducing environment—to ensure the metal surfaces remain free of oxides, allowing for maximum densification and material strength.
A laboratory tube furnace enables the sintering of Cu-Al2O3 composites by facilitating atomic diffusion under strictly controlled atmospheres. This process eliminates surface oxides and promotes strong interfacial bonding, resulting in a dense, high-performance material.

Facilitating Atomic Diffusion and Densification
The Role of High-Temperature Thermal Energy
The sintering process requires a high-temperature environment, generally maintained near 900 °C, to initiate the movement of atoms across particle boundaries. This thermal energy allows the individual copper and alumina particles to bond into a single, cohesive matrix without melting the entire mass.
Achieving Material Strengthening
As the furnace maintains a consistent temperature field, it promotes densification, a process where the porosity of the composite is reduced. This leads to significantly improved mechanical properties and structural integrity in the final Cu-Al2O3 component.
Ensuring Microstructural Uniformity
The design of the furnace tube ensures a uniform temperature field throughout the material volume. This consistency is vital for achieving a stable microstructure, which directly impacts the distribution of Al2O3 particles within the copper matrix and prevents localized defects.
Atmospheric Control and Surface Chemistry
The Function of Reducing Atmospheres
Operating the tube furnace under a hydrogen reducing atmosphere is critical for managing surface chemistry. This atmosphere actively removes residual oxide films from the copper powder surfaces, which would otherwise act as barriers to effective bonding.
Prevention of Secondary Oxidation
Because aluminum is highly reactive to oxygen, the furnace must provide a strictly sealed environment to prevent secondary oxidation. Utilizing a vacuum or an inert atmosphere like argon ensures that the composite remains pure and prevents the formation of undesirable intermetallic phases.
Promoting Interfacial Bonding
By maintaining a low-oxygen or reducing environment, the furnace facilitates diffusion bonding at the interface of the copper and alumina. This ensures a strong mechanical and chemical connection between the ceramic reinforcement and the metallic matrix.
Understanding the Trade-offs
Atmospheric Complexity vs. Safety
Using a hydrogen reducing atmosphere is highly effective for removing oxides, but it requires rigorous safety protocols to manage the flammability of the gas. Engineers must weigh the superior cleaning capability of hydrogen against the simpler, safer use of inert gases like argon, which may not remove existing oxides as effectively.
Vacuum vs. Positive Pressure
A high-vacuum environment (often around 0.09 mbar) is excellent for preventing oxidation but can lead to the volatilization of certain elements at high temperatures. In contrast, a positive pressure inert atmosphere is easier to maintain but requires high-purity gas to avoid trace contamination.
Sintering Time and Grain Growth
Longer sintering times in the furnace can improve densification, but they also risk excessive grain growth. Oversized grains can diminish the mechanical advantages of the Al2O3 reinforcement, requiring a precise balance between processing duration and desired material hardness.
Applying Furnace Parameters to Your Project
When utilizing a tube furnace for Cu-Al2O3 composite fabrication, your operational choices should align with your specific performance requirements.
- If your primary focus is Maximum Conductivity: Use a hydrogen reducing atmosphere at 900 °C to ensure all copper oxide is removed, as residual oxides significantly increase electrical resistance.
- If your primary focus is High Mechanical Hardness: Prioritize a vacuum environment to prevent secondary oxidation of the aluminum components, ensuring the strongest possible bond between the ceramic and metal phases.
- If your primary focus is Scalability and Safety: Opt for a high-purity argon atmosphere, which provides sufficient protection for many applications while simplifying the gas handling and safety infrastructure.
The laboratory tube furnace remains the definitive tool for transforming composite powders into high-performance engineering materials through the precise synchronization of heat and chemistry.
Summary Table:
| Parameter | Role in Cu-Al2O3 Sintering | Key Benefit |
|---|---|---|
| Temperature (~900°C) | Initiates atomic diffusion and particle bonding | Achieves densification and structural integrity |
| Hydrogen Atmosphere | Removes surface oxide films from copper | Maximizes electrical conductivity and bonding |
| Vacuum/Inert Gas | Prevents secondary oxidation of aluminum | Maintains material purity and prevents defects |
| Thermal Uniformity | Ensures consistent heat across the composite | Creates a stable microstructure and prevents localized defects |
Optimize Your Composite Materials with KINTEK
Unlock the full potential of your Cu-Al2O3 composites with high-precision thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers specialized Tube, Vacuum, and CVD systems designed to provide the rigorous atmospheric control and temperature uniformity required for advanced material sintering.
Whether you need to eliminate oxides with hydrogen reduction or achieve high-vacuum purity, our customizable lab high-temp furnaces are built to meet your unique research needs. Contact KINTEK today to discuss your sintering requirements and see how our expertise can drive your material performance forward.
Visual Guide
References
- Tawfik M. Ahmed. Development and characterization of Cu-Al2O3 metal matrix composites through powder metallurgy techniques. DOI: 10.33545/26646536.2025.v7.i2a.137
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the primary function of a high-temperature tube furnace in h-BN preparation? Achieve Clean Surface Activation
- What are the pros and cons of vertical tube furnaces? Precision vs. Capacity for Your Lab
- What is the role of a laboratory tube furnace in the carbonization of peanut shells? Master Biochar Preparation
- What features enhance the thermal efficiency of split tube furnaces? Boost Performance with Key Design Elements
- Why is a tube furnace with precise temperature control critical for the preparation of Palladium Borosulfates?
- How does a horizontal tube furnace ensure experimental safety and accuracy during the thermal dehydrogenation of Ca(AlH4)2?
- What is the role of high-temperature tube furnaces in the post-processing of graphite oxide nanostructures?
- What are the technical specifications of a Drop Tube Furnace? Optimize Your High-Temperature Conversion Experiments



















