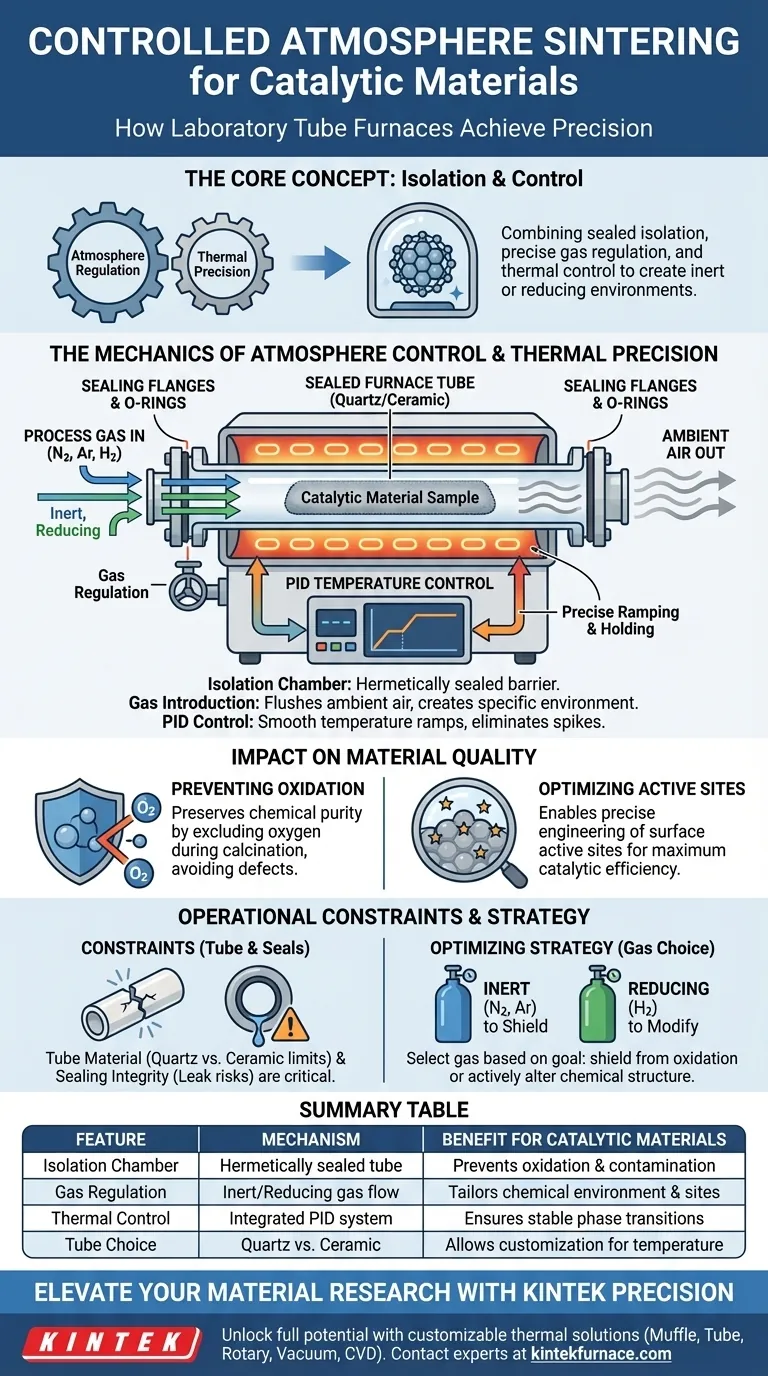
A laboratory tube furnace achieves controlled atmosphere sintering by isolating the catalytic material within a hermetically sealed quartz or ceramic tube and introducing specific process gases to dictate the chemical environment. This setup prevents ambient oxygen from interfering with the sample while an integrated PID system manages the thermal profile with high precision.
By combining a sealed isolation chamber with precise gas regulation, the tube furnace creates a specific inert or reducing environment. This allows for the exact manipulation of crystal structures and surface active sites, ensuring the final catalytic material performs as designed without oxidation defects.

The Mechanics of Atmosphere Control
To understand how these furnaces facilitate high-quality sintering, we must look at how they manage the physical environment around the sample.
The Isolation Chamber
The core of the system is the sealed furnace tube, typically made of quartz or ceramic.
This tube acts as the primary barrier. It completely isolates the catalytic material from the laboratory's ambient air.
Gas Introduction and Regulation
Once sealed, the system introduces specific process gases such as nitrogen, argon, or hydrogen.
These gases flush out any remaining air. This replaces the standard atmosphere with a strictly controlled inert or reducing environment.
The Role of Thermal Precision
Atmosphere alone is not enough; the relationship between temperature and gas flow is critical for catalytic preparation.
PID Temperature Control
The furnace utilizes an integrated PID (Proportional-Integral-Derivative) control system.
This ensures the temperature ramps up and holds with extreme accuracy. It eliminates temperature spikes that could damage sensitive material structures.
Managing Phase Transitions
Catalytic materials often undergo complex phase transitions at high temperatures.
The PID system ensures these transitions occur smoothly. It stabilizes the thermal environment so the material settles into the desired crystalline state.
Impact on Material Quality
The ultimate goal of this equipment is to define the chemical and physical properties of the catalyst.
Preventing Oxidation
During processes like calcination, many materials are prone to unwanted oxidation.
The controlled gas flow prevents oxygen from reacting with the sample. This preserves the chemical purity required for the catalyst to function.
Optimizing Active Sites
Catalytic performance depends on the availability of surface active sites.
By controlling both the atmosphere and the heat, the furnace enables precise engineering of these sites. This directly correlates to the efficiency of the final catalytic product.
Understanding the Operational Constraints
While tube furnaces are powerful tools, there are trade-offs inherent to their design that affect operation.
Tube Material Limitations
The choice between quartz and ceramic tubes dictates your maximum operating temperature.
Quartz provides visibility but has a lower thermal limit. Ceramic can withstand higher heat but is opaque and more susceptible to thermal shock if cooled too quickly.
Sealing Integrity Risks
The effectiveness of the process relies entirely on the quality of the seals.
Even a microscopic leak can introduce enough oxygen to compromise the inert environment. Regular inspection of O-rings and flanges is a non-negotiable maintenance requirement.
Optimizing Your Sintering Strategy
To get the most out of your laboratory tube furnace, align your gas choice with your specific material goals.
- If your primary focus is preventing oxidation: Prioritize inert gases like nitrogen or argon to simply shield the material during high-temperature exposure.
- If your primary focus is modifying chemical structure: Utilize reducing gases like hydrogen to actively strip oxygen atoms and alter the material's stoichiometry.
Success in catalytic preparation comes from the precise synchronization of a sealed environment, gas chemistry, and thermal regulation.
Summary Table:
| Feature | Mechanism | Benefit for Catalytic Materials |
|---|---|---|
| Isolation Chamber | Hermetically sealed quartz or ceramic tube | Prevents oxidation and atmospheric contamination |
| Gas Regulation | Introduction of inert (N2/Ar) or reducing (H2) gases | Tailors chemical environment and active sites |
| Thermal Control | Integrated PID system with precise ramping | Ensures stable phase transitions and crystal structure |
| Tube Choice | Quartz (visible/low temp) vs Ceramic (high temp) | Allows customization based on thermal requirements |
Elevate Your Material Research with KINTEK Precision
Unlock the full potential of your catalytic preparation with KINTEK’s advanced thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific laboratory requirements. Whether you need to optimize surface active sites or maintain strict inert environments, our lab high-temp furnaces provide the reliability you need.
Ready to engineer superior materials? Contact our technical experts today to find the perfect furnace for your application.
Visual Guide
References
- Jianjun Ma, Qiuhong Zhou. Galvanic Displacement Engineered Pt/Co₃O₄‐CeO₂ for High‐Efficiency Toluene Elimination at Low Temperature. DOI: 10.1002/slct.202405496
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is the function of a cold tube furnace for magnesium extraction? Achieve Ultra-Pure Metal with Vacuum Evaporation
- What is a tube furnace and its main characteristics? Discover Precision Heating for Your Lab
- How does a high-temperature tube furnace combustion system function in food waste analysis? Master Ultimate Analysis
- How does a dual-zone tube furnace facilitate the synthesis of CrSBr single crystals? Master the CVT Process
- How does a platinum tube heating device assist in studying tungsten work function? Precision Oxygen Purification
- Why is a high-vacuum tube furnace necessary for TMD annealing? Protect Your Monolayers from Oxidative Ablation
- What is the basic working principle of a multi gradient experimental tube furnace? Unlock Precise Temperature Control for Advanced Experiments
- How did the tube furnace originate and where is it commonly used today? Discover Its Evolution and Modern Applications



















