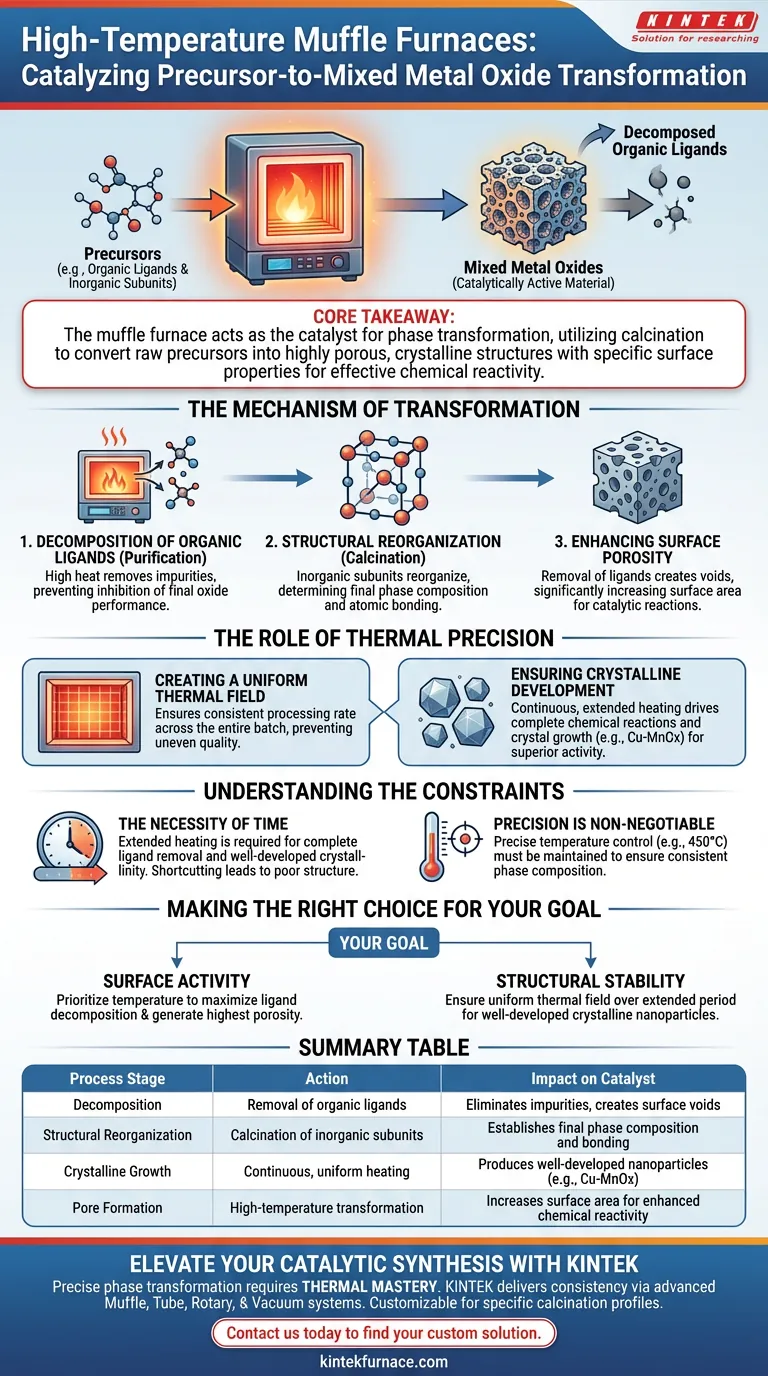
High-temperature muffle furnaces drive the synthesis of mixed metal oxides by subjecting precursors to precise thermal treatment, often around 450 degrees Celsius. This controlled environment facilitates two critical actions: the complete decomposition of organic ligands and the structural reorganization of inorganic subunits. The result is a catalytically active material optimized for tasks such as degrading organic dyes or reducing heavy metal ions.
Core Takeaway: The muffle furnace acts as the catalyst for phase transformation, utilizing calcination to convert raw precursors into highly porous, crystalline structures with the specific surface properties required for effective chemical reactivity.
The Mechanism of Transformation
Decomposition of Organic Ligands
The first critical function of the furnace is purification. By maintaining high temperatures, the furnace induces the thorough decomposition of organic ligands present in the precursor materials.
Removing these organic components is essential. It eliminates impurities that would otherwise inhibit the performance of the final mixed metal oxide.
Structural Reorganization
Simultaneously, the heat triggers a fundamental change in the material's architecture. Inorganic subunits begin to reorganize, a process known as calcination.
This step determines the final phase composition of the material. It dictates how the metal atoms bond and arrange themselves to form the desired oxide.
Enhancing Surface Porosity
The physical changes driven by the furnace significantly increase surface porosity. As ligands are removed and the structure settles, voids are created within the material.
This porosity is the key to catalytic performance. A porous surface provides a larger area for chemical reactions to occur, directly enhancing the material's ability to degrade dyes or reduce heavy metals.
The Role of Thermal Precision
Creating a Uniform Thermal Field
An industrial-grade muffle furnace provides a constant and uniform thermal field. This consistency is vital, particularly when heating complex setups like high-pressure hydrothermal reactors.
Uniformity ensures that the entire batch of precursor material processes at the same rate. This prevents uneven quality in the final product.
Ensuring Crystalline Development
The furnace's ability to maintain continuous heating for extended periods ensures chemical reactions proceed to absolute completion. This duration is critical for crystal growth.
Controlled heating leads to the formation of well-developed crystalline nanoparticles, such as Cu-MnOx. High crystallinity is directly linked to superior catalytic activity.
Understanding the Constraints
The Necessity of Time
The conversion process is not instantaneous. To achieve well-developed crystallinity and complete ligand removal, the material requires continuous heating over extended periods.
Shortcutting the heating duration can result in incomplete chemical reactions. This yields a material with poor structural integrity and lower catalytic potential.
Precision is Non-Negotiable
The effectiveness of the process relies entirely on a precise temperature control system. The specific temperature (e.g., 450 degrees Celsius) must be maintained without fluctuation.
If the temperature varies, the phase composition may become inconsistent. This jeopardizes the uniformity of the final mixed metal oxides.
Making the Right Choice for Your Goal
To maximize the efficacy of your synthesis, align your furnace parameters with your specific material requirements:
- If your primary focus is surface activity: Prioritize temperature settings that maximize the decomposition of organic ligands to generate the highest possible porosity.
- If your primary focus is structural stability: Ensure the furnace maintains a uniform thermal field over an extended period to foster well-developed crystalline nanoparticles.
By strictly controlling the thermal environment, you ensure the transition from precursor to potent catalyst is both complete and repeatable.
Summary Table:
| Process Stage | Action | Impact on Catalyst |
|---|---|---|
| Decomposition | Removal of organic ligands | Eliminates impurities and creates surface voids |
| Structural Reorganization | Calcination of inorganic subunits | Establishes the final phase composition and bonding |
| Crystalline Growth | Continuous, uniform heating | Produces well-developed nanoparticles (e.g., Cu-MnOx) |
| Pore Formation | High-temperature transformation | Increases surface area for enhanced chemical reactivity |
Elevate Your Catalytic Synthesis with KINTEK
Precise phase transformation requires more than just heat—it requires thermal mastery. KINTEK delivers the consistency your research demands through our advanced range of Muffle, Tube, Rotary, and Vacuum systems.
Backed by expert R&D and manufacturing, our high-temp furnaces are fully customizable to handle specific calcination profiles, ensuring complete ligand removal and superior crystalline development for your mixed metal oxides.
Ready to optimize your material performance? Contact us today to find your custom solution.
Visual Guide
References
- Zi‐Qing Liu, Bao‐Li Fei. Mixed Metal Oxide Derived from Polyoxometalate-Based Metal–Organic Framework as a Bi-Functional Heterogeneous Catalyst for Wastewater Treatment. DOI: 10.3390/catal15010076
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What precautions should be taken while using muffle furnace? Ensure Complete Safety and Longevity
- What is the role of Muffle or Tube furnaces in carbon nitride preparation? Optimize Your Thermal Polymerization
- What is the function of insulating material in a muffle furnace? Unlock Efficiency and Safety in Your Lab
- What are the main advantages of using a muffle furnace? Achieve Precise, Contamination-Free Heating
- Why do conventional furnaces require long holding times for BCZY712 sintering? Overcome Heat Efficiency Challenges
- How is a laboratory high-temperature muffle furnace utilized to achieve the specific crystalline structure of LaFeO3 catalysts?
- How are muffle furnaces utilized in high-temperature sintering within the pharmaceutical industry? Unlock Precision in Drug Delivery and Implants
- What are the key aspects to consider when choosing a muffle furnace? Ensure Optimal Performance and Safety



















